NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease
- PMID: 11489943
- PMCID: PMC2193462
- DOI: 10.1084/jem.194.3.235
NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease
Erratum in
- J Exp Med 2001 Sep 3;194(5):following 703
Abstract
In humans, natural killer (NK) cell function is regulated by a series of receptors and coreceptors with either triggering or inhibitory activity. Here we describe a novel 60-kD glycoprotein, termed NTB-A, that is expressed by all human NK, T, and B lymphocytes. Monoclonal antibody (mAb)-mediated cross-linking of NTB-A results in the induction of NK-mediated cytotoxicity. Similar to 2B4 (CD244) functioning as a coreceptor in the NK cell activation, NTB-A also triggers cytolytic activity only in NK cells expressing high surface densities of natural cytotoxicity receptors. This suggests that also NTB-A may function as a coreceptor in the process of NK cell activation. Molecular cloning of the cDNA coding for NTB-A molecule revealed a novel member of the immunoglobulin superfamily belonging to the CD2 subfamily. NTB-A is characterized, in its extracellular portion, by a distal V-type and a proximal C2-type domain and by a cytoplasmic portion containing three tyrosine-based motifs. NTB-A undergoes tyrosine phosphorylation and associates with the Src homology 2 domain-containing protein (SH2D1A) as well as with SH2 domain-containing phosphatases (SHPs). Importantly, analysis of NK cells derived from patients with X-linked lymphoproliferative disease (XLP) showed that the lack of SH2D1A protein profoundly affects the function not only of 2B4 but also of NTB-A. Thus, in XLP-NK cells, NTB-A mediates inhibitory rather than activating signals. These inhibitory signals are induced by the interaction of NTB-A with still undefined ligands expressed on Epstein-Barr virus (EBV)-infected target cells. Moreover, mAb-mediated masking of NTB-A can partially revert this inhibitory effect while a maximal recovery of target cell lysis can be obtained when both 2B4 and NTB-A are simultaneously masked. Thus, the altered function of NTB-A appears to play an important role in the inability of XLP-NK cells to kill EBV-infected target cells.
Figures
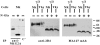









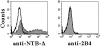

References
-
- Moretta A., Bottino C., Vitale M., Pende D., Biassoni R., Mingari M.C., Moretta L. Receptors for HLA-class I molecules in human natural killer cells. Annu. Rev. Immunol. 1996;14:619–648. - PubMed
-
- Lanier L.L. NK cell receptors. Annu. Rev. Immunol. 1998;16:359–393. - PubMed
-
- Long E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 1999;17:875–904. - PubMed
-
- Moretta A., Bottino C., Vitale M., Pende D., Cantoni C., Mingari M.C., Biassoni R., Moretta L. Activating receptors and co-receptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001;19:197–223. - PubMed
-
- Garrido F., Ruiz-Cabello F., Cabrera T., Pérez-Villar J.J., Lòpez-Botet M., Duggan-Keen M., Stern P.L. Implications for immunosurveillance of altered HLA-class I phenotypes in human tumours. Immunol. Today. 1997;18:89–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

