Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module
- PMID: 11489945
- PMCID: PMC2193464
- DOI: 10.1084/jem.194.3.255
Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module
Abstract
In latently infected B lymphocytes, the Epstein-Barr virus (EBV) suppresses signal transduction from the antigen receptor through expression of the integral latent membrane protein 2A (LMP2A). At the same time, LMP2A triggers B cell survival by a yet uncharacterized maintenance signal that is normally provided by the antigen receptor. The molecular mechanisms are unknown as LMP2A-regulated signaling cascades have not been described so far. Using a novel mouse model we have identified the intracellular adaptor protein Src homology 2 (SH2) domain-containing leukocyte protein (SLP)-65 as a critical downstream effector of LMP2A in vivo. Biochemical analysis of the underlying signaling pathways revealed that EBV infection causes constitutive tyrosine phosphorylation of one of the two SLP-65 isoforms and complex formation between SLP-65 and the protooncoprotein CrkL (CT10 regulator of kinase like). This leads to antigen receptor-independent phosphorylation of Cbl (Casitas B lineage lymphoma) and C3G. In contrast, phospholipase C-gamma2 (PLC-gamma2) activation is completely blocked. Our data show that in order to establish a latent EBV infection, LMP2A selectively activates or represses SLP-65-regulated signaling pathways.
Figures






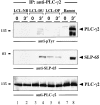
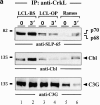

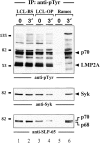
References
-
- Rickinson A.B., Kieff E. Epstein-Barr viruses. In: Fields B.N., Knipe D.M., Howely P.M., editors. Fields Virology. Lippincott-Raven; Philadelphia, PA: 1996. pp. 2397–2446.
-
- Longnecker R. Molecular biology of Epstein-Barr virus. In: McCane D., editor. Human Tumor Viruses. American Society for Microbiology; Washington, D.C.: 1998. pp. 133–172.
-
- Kieff E. Epstein-Barr virus and its replication. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fundamental Virology. Lippincott-Raven; Philadelphia, PA: 1996. pp. 1009–1162.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

