p62(dok), a negative regulator of Ras and mitogen-activated protein kinase (MAPK) activity, opposes leukemogenesis by p210(bcr-abl)
- PMID: 11489947
- PMCID: PMC2193466
- DOI: 10.1084/jem.194.3.275
p62(dok), a negative regulator of Ras and mitogen-activated protein kinase (MAPK) activity, opposes leukemogenesis by p210(bcr-abl)
Abstract
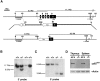
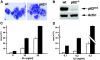
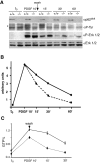
p62(dok) has been identified as a substrate of many oncogenic tyrosine kinases such as the chronic myelogenous leukemia (CML) chimeric p210(bcr-abl) oncoprotein. It is also phosphorylated upon activation of many receptors and cytoplamic tyrosine kinases. However, the biological functions of p62(dok) in normal cell signaling as well as in p210(bcr-abl) leukemogenesis are as yet not fully understood. Here we show, in hemopoietic and nonhemopoietic cells derived from p62(dok)-(/)- mice, that the loss of p62(dok) results in increased cell proliferation upon growth factor treatment. Moreover, Ras and mitogen-activated protein kinase (MAPK) activation is markedly sustained in p62(dok)-(/)- cells after the removal of growth factor. However, p62(dok) inactivation does not affect DNA damage and growth factor deprivation-induced apoptosis. Furthermore, p62(dok) inactivation causes a significant shortening in the latency of the fatal myeloproliferative disease induced by retroviral-mediated transduction of p210(bcr-abl) in bone marrow cells. These data indicate that p62(dok) acts as a negative regulator of growth factor-induced cell proliferation, at least in part through downregulating Ras/MAPK signaling pathway, and that p62(dok) can oppose leukemogenesis by p210(bcr-abl).
Figures
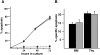



References
-
- de Klein A., van Kessel A.G., Grosveld G., Bartram C.R., Hagemeijer A., Bootsma D., Spurr N.K., Heisterkamp N., Groffen J., Stephenson J.R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. - PubMed
-
- Lugo T.G., Pendergast A.M., Muller A.J., Witte O.N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. - PubMed
-
- Clarkson B., Strife A. Linkage of proliferative and maturational abnormalities in chronic myelogenous leukemia and relevance to treatment. Leukemia. 1993;7:1683–1721. - PubMed
-
- Carpino N., Wisniewski D., Strife A., Marshak D., Kobayashi R., Stillman B., Clarkson B. p62(dok)a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. - PubMed
-
- Yamanashi Y., Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

