CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes
- PMID: 11489950
- PMCID: PMC2193465
- DOI: 10.1084/jem.194.3.313
CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes
Abstract
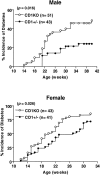
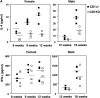
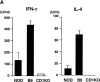
NK T cells are a unique subset of T cells that recognize lipid antigens presented by CD1d. After activation, NK T cells promptly produce large amounts of cytokines, which may modulate the upcoming immune responses. Previous studies have documented an association between decreased numbers of NK T cells and the progression of some autoimmune diseases, suggesting that NK T cells may control the development of autoimmune diseases. To investigate the role of NK T cells in autoimmune diabetes, we crossed CD1 knockout (CD1KO) mutation onto the nonobese diabetic (NOD) genetic background. We found that male CD1KO NOD mice exhibited significantly higher incidence and earlier onset of diabetes compared with the heterozygous controls. The diabetic frequencies in female mice showed a similar pattern; however, the differences were less profound between female CD1KO and control mice. Early treatment of NOD mice with alpha-galactosylceramide, a potent NK T cell activator, reduced the severity of autoimmune diabetes in a CD1-dependent manner. Our results not only suggest a protective role of CD1-restricted NK T cells in autoimmune diabetes but also reveal a causative link between the deficiency of NK T cells and the induction of insulin-dependent diabetes mellitus.
Figures




References
-
- Kikutani H., Makino S. The murine autoimmune diabetes modelNOD and related strains. Adv. Immunol. 1992;51:285–322. - PubMed
-
- Miller B.J., Appel M.C., O'Neil J.J., Wicker L.S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J. Immunol. 1988;140:52–58. - PubMed
-
- Yagi H., Matsumoto M., Kunimoto K., Kawaguchi J., Makino S., Harada M. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur. J. Immunol. 1992;22:2387–2393. - PubMed
-
- Katz J.D., Benoist C., Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

