Absence of Mn-centered oxidation in the S(2) --> S(3) transition: implications for the mechanism of photosynthetic water oxidation
- PMID: 11493054
- PMCID: PMC3965774
- DOI: 10.1021/ja004307+
Absence of Mn-centered oxidation in the S(2) --> S(3) transition: implications for the mechanism of photosynthetic water oxidation
Abstract
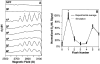
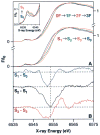
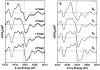
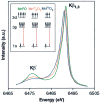
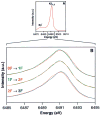
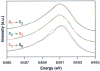
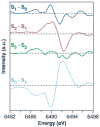
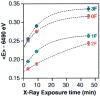
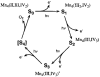
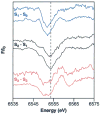
A key question for the understanding of photosynthetic water oxidation is whether the four oxidizing equivalents necessary to oxidize water to dioxygen are accumulated on the four Mn ions of the oxygen-evolving complex (OEC), or whether some ligand-centered oxidations take place before the formation and release of dioxygen during the S(3) --> [S(4)] --> S(0) transition. Progress in instrumentation and flash sample preparation allowed us to apply Mn Kbeta X-ray emission spectroscopy (Kbeta XES) to this problem for the first time. The Kbeta XES results, in combination with Mn X-ray absorption near-edge structure (XANES) and electron paramagnetic resonance (EPR) data obtained from the same set of samples, show that the S(2) --> S(3) transition, in contrast to the S(0) --> S(1) and S(1) --> S(2) transitions, does not involve a Mn-centered oxidation. On the basis of new structural data from the S(3)-state, manganese mu-oxo bridge radical formation is proposed for the S(2) --> S(3) transition, and three possible mechanisms for the O-O bond formation are presented.
Figures


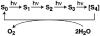
References
-
- Debus RJ. Biochim Biophys Acta. 1992;1102:269–352. - PubMed
-
- Britt RD. In: Oxygenic Photosynthesis: The Light Reactions. Ort DR, Yocum CF, editors. Vol. 4. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1996. pp. 137–164.
-
- Diner BA, Babcock GT. In: Oxygenic Photosynthesis: The Light Reactions. Ort DR, Yocum CF, editors. Vol. 4. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1996. pp. 213–247.
-
- Kok B, Forbush B, McGloin M. Photochem Photobiol. 1970;11:457–475. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

