A novel mammalian receptor for the evolutionarily conserved type II GnRH
- PMID: 11493674
- PMCID: PMC55504
- DOI: 10.1073/pnas.141048498
A novel mammalian receptor for the evolutionarily conserved type II GnRH
Abstract
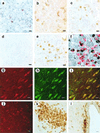
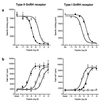
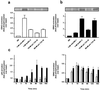
Mammalian gonadotropin-releasing hormone (GnRH I: pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) stimulates pituitary gonadotropin secretion, which in turn stimulates the gonads. Whereas a hypothalamic form of GnRH of variable structure (designated type I) had been shown to regulate reproduction through a cognate type I receptor, it has recently become evident that most vertebrates have one or two other forms of GnRH. One of these, designated type II GnRH (GnRH II: pGlu-His-Ser-His-Gly-Trp-Tyr-Pro-Gly-NH2), is conserved from fish to man and is widely distributed in the brain, suggesting important neuromodulatory functions such as regulating K+ channels and stimulating sexual arousal. We now report the cloning of a type II GnRH receptor from marmoset cDNA. The receptor has only 41% identity with the type I receptor and, unlike the type I receptor, has a carboxyl-terminal tail. The receptor is highly selective for GnRH II. As with the type I receptor, it couples to G(alpha)q/11 and also activates extracellular signal-regulated kinase (ERK1/2) but differs in activating p38 mitogen activated protein (MAP) kinase. The type II receptor is more widely distributed than the type I receptor and is expressed throughout the brain, including areas associated with sexual arousal, and in diverse non-neural and reproductive tissues, suggesting a variety of functions. Surprisingly, the type II receptor is expressed in the majority of gonadotropes. The presence of two GnRH receptors in gonadotropes, together with the differences in their signaling, suggests different roles in gonadotrope functioning.
Figures


Similar articles
-
Isolation and characterisation of the marmoset gonadotrophin releasing hormone receptor: Ser(140) of the DRS motif is substituted by Phe.J Endocrinol. 1999 Dec;163(3):447-56. doi: 10.1677/joe.0.1630447. J Endocrinol. 1999. PMID: 10588818
-
A gonadotropin-releasing hormone (GnRH) receptor specific for GnRH II in primates.Biochem Biophys Res Commun. 2001 Apr 13;282(4):1012-8. doi: 10.1006/bbrc.2001.4678. Biochem Biophys Res Commun. 2001. PMID: 11352653
-
Bovine and ovine gonadotropin-releasing hormone (GnRH)-II ligand precursors and type II GnRH receptor genes are functionally inactivated.Endocrinology. 2006 Nov;147(11):5041-51. doi: 10.1210/en.2006-0222. Epub 2006 Aug 17. Endocrinology. 2006. PMID: 16916952
-
GnRH II and type II GnRH receptors.Trends Endocrinol Metab. 2003 Jan;14(1):35-43. doi: 10.1016/s1043-2760(02)00016-4. Trends Endocrinol Metab. 2003. PMID: 12475610 Review.
-
GnRHs and GnRH receptors.Anim Reprod Sci. 2005 Aug;88(1-2):5-28. doi: 10.1016/j.anireprosci.2005.05.032. Anim Reprod Sci. 2005. PMID: 16140177 Review.
Cited by
-
Contemporary pharmacological manipulation in assisted reproduction.Drugs. 2004;64(3):297-322. doi: 10.2165/00003495-200464030-00005. Drugs. 2004. PMID: 14871171 Review.
-
Gonadotropin-Releasing Hormone Receptors in Prostate Cancer: Molecular Aspects and Biological Functions.Int J Mol Sci. 2020 Dec 14;21(24):9511. doi: 10.3390/ijms21249511. Int J Mol Sci. 2020. PMID: 33327545 Free PMC article. Review.
-
Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains.Cell Mol Neurobiol. 2002 Dec;22(5-6):589-609. doi: 10.1023/a:1021888420271. Cell Mol Neurobiol. 2002. PMID: 12838906 Free PMC article. Review.
-
Gonadotropin-releasing hormone II: a multi-purpose neuropeptide.Integr Comp Biol. 2008 Nov;48(5):588-95. doi: 10.1093/icb/icn018. Epub 2008 Apr 19. Integr Comp Biol. 2008. PMID: 21669818 Free PMC article.
-
Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the invasiveness of endometrial cancer cells through the GnRH-I receptor and mitogen-activated protein kinase (MAPK)-dependent activation of matrix metalloproteinase (MMP)-2.BMC Cancer. 2013 Jun 20;13:300. doi: 10.1186/1471-2407-13-300. BMC Cancer. 2013. PMID: 23786715 Free PMC article.
References
-
- Millar R P, King J A, Davidson J S, Milton R C. S Afr Med J. 1987;72:748–755. - PubMed
-
- Conn P M, Crowley W F., Jr N Engl J Med. 1991;324:93–103. - PubMed
-
- Conn P M, Crowley W F., Jr Annu Rev Med. 1994;45:391–405. - PubMed
-
- Nieschlag E, Behre H M, Weinbauer G F. In: Spermatogenesis-Fertilization-Contraception: Molecular, Cellular and Endocrine Events in Male Reproduction. Nieschlag E, Habenicht U-F, editors. Berlin: Springer; 1992. pp. 447–501.
-
- Fraser H M. Br Med Bull. 1993;49:62–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

