Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion
- PMID: 11493675
- PMCID: PMC55543
- DOI: 10.1073/pnas.151098198
Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion
Abstract
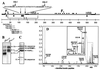
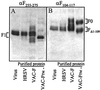
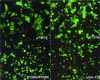
Preparations of purified full-length fusion (F) protein of human respiratory syncytial virus (HRSV) expressed in recombinant vaccinia-F infected cells, or of an anchorless mutant (F(TM(-))) lacking the C-terminal 50 amino acids secreted from vaccinia-F(TM(-))-infected cells contain a minor polypeptide that is an intermediate product of proteolytic processing of the F protein precursor F0. N-terminal sequencing of the intermediate demonstrated that it is generated by cleavage at a furin-motif, residues 106-109 of the F sequence. By contrast, the F1 N terminus derives from cleavage at residue 137 of F0 which is also C-terminal to a furin recognition site at residues 131-136. Site-directed mutagenesis indicates that processing of F0 protein involves independent cleavage at both sites. Both cleavages are required for the F protein to be active in membrane fusion as judged by syncytia formation, and they allow changes in F structure from cone- to lollipop-shaped spikes and the formation of rosettes by anchorless F.
Figures

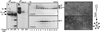
References
-
- Collins P L, Chanock R M, Murphy B R. In: Fields Virology. Fields BN, Knipe DM, Howley PM, editors. Philadelphia: Lippincott; 1996. pp. 1313–1351.
-
- Han L L, Alexander J P, Anderson L J. J Infect Dis. 1999;179:25–30. - PubMed
-
- Levine S, Klaiber-Franco R, Paradiso P R. J Gen Virol. 1987;68:2521–2524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

