The yeast mutant vps5Delta affected in the recycling of Golgi membrane proteins displays an enhanced vacuolar Mg2+/H+ exchange activity
- PMID: 11493679
- PMCID: PMC55508
- DOI: 10.1073/pnas.161215198
The yeast mutant vps5Delta affected in the recycling of Golgi membrane proteins displays an enhanced vacuolar Mg2+/H+ exchange activity
Abstract
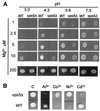
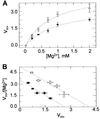
Growth of the yeast vacuolar protein-sorting mutant vps5Delta affected in the endosome-to-Golgi retromer complex was more sensitive to Mg2+-limiting conditions than was the growth of the wild-type (WT) strain. This sensitivity was enhanced at acidic pH. The vps5Delta strain was also sensitive to Al3+, known to inhibit Mg2+ uptake in yeast cells. In contrast, it was found to be resistant to Ni2+ and Co2+, two cytotoxic analogs of Mg2+. Resistance to Ni2+ did not seem to result from the alteration of plasma-membrane transport properties because mutant and WT cells displayed similar Ni2+ uptake. After plasma-membrane permeabilization, intracellular Ni2+ uptake in vps5Delta cells was 3-fold higher than in WT cells, which is consistent with the implication of the vacuole in the observed phenotypes. In reconstituted vacuolar vesicles prepared from vps5Delta, the rates of H+ exchange with Ni2+, Co2+, and Mg2+ were increased (relative to WT) by 170%, 130%, and 50%, respectively. The rates of H+ exchange with Ca2+, Cd2+, and K+ were similar in both strains, as were alpha-mannosidase and H+-ATPase activities, and SDS/PAGE patterns of vacuolar proteins. Among 14 other vacuolar protein-sorting mutants tested, only the 8 mutants affected in the recycling of trans-Golgi network membrane proteins shared the same Ni2+ resistance phenotype as vps5Delta. It is proposed that a trans-Golgi network Mg2+/H+ exchanger, mislocalized to vps5Delta vacuole, could be responsible for the phenotypes observed in vivo and in vitro.
Figures



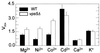

References
-
- Lemmon S K, Traub L M. Curr Opin Cell Biol. 2000;12:457–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

