Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization
- PMID: 11493690
- PMCID: PMC55505
- DOI: 10.1073/pnas.171160298
Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization
Abstract
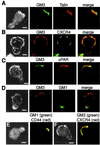
Redistribution of specialized molecules in migrating cells develops asymmetry between two opposite cell poles, the leading edge and the uropod. We show that acquisition of a motile phenotype in T lymphocytes results in the asymmetric redistribution of ganglioside GM3- and GM1-enriched raft domains to the leading edge and to the uropod, respectively. This segregation to each cell pole parallels the specific redistribution of membrane proteins associated to each raft subfraction. Our data suggest that raft partitioning is a major determinant for protein redistribution in polarized T cells, as ectopic expression of raft-associated proteins results in their asymmetric redistribution, whereas non-raft-partitioned mutants of these proteins are distributed homogeneously in the polarized cell membrane. Both acquisition of a migratory phenotype and SDF-1alpha-induced chemotaxis are cholesterol depletion-sensitive. Finally, GM3 and GM1 raft redistribution requires an intact actin cytoskeleton, but is insensitive to microtubule disruption. We propose that membrane protein segregation not only between raft and nonraft domains but also between distinct raft subdomains may be an organizational principle that mediates redistribution of specialized molecules needed for T cell migration.
Figures






Comment in
-
Flotillas of lipid rafts fore and aft.Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9471-3. doi: 10.1073/pnas.181353098. Proc Natl Acad Sci U S A. 2001. PMID: 11504934 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

