Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2
- PMID: 11493697
- PMCID: PMC55530
- DOI: 10.1073/pnas.171269898
Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2
Abstract
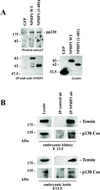
Juvenile nephronophthisis type 1 is caused by mutations of NPHP1, the gene encoding for nephrocystin. The function of nephrocystin is presently unknown, but the presence of a Src homology 3 domain and its recently described interaction with p130(Cas) suggest that nephrocystin is part of the focal adhesion signaling complex. We generated a nephrocystin-specific antiserum and analyzed the interaction of native nephrocystin with endogenous proteins. Immunoprecipitation of nephrocystin revealed that nephrocystin forms protein complexes with p130(Cas), proline-rich tyrosine kinase 2 (Pyk2), and tensin, indicating that these proteins participate in a common signaling pathway. Expression of nephrocystin resulted in phosphorylation of Pyk2 on tyrosine 402 as well as activation of downstream mitogen-activated protein kinases, such as ERK1 and ERK2. Our findings suggest that nephrocystin helps to recruit Pyk2 to cell matrix adhesions, thereby initiating phosphorylation of Pyk2 and Pyk2-dependent signaling. A lack of functional nephrocystin may compromise Pyk2 signaling in a subset of renal epithelial cells.
Figures




References
-
- Hildebrandt F, Otto E. J Am Soc Nephrol. 2000;11:1753–1761. - PubMed
-
- Waldherr R, Lennert T, Weber H P, Fodisch H J, Scharer K. Virchows Arch Pathol Anat Physiol Klin Med. 1982;394:235–254. - PubMed
-
- Hildebrandt F, Otto E, Rensing C, Nothwang H G, Vollmer M, Adolphs J, Hanusch H, Brandis M. Nat Genet. 1997;17:149–153. - PubMed
-
- Saunier S, Calado J, Heilig R, Silbermann F, Benessy F, Morin G, Konrad M, Broyer M, Gubler M C, Weissenbach J, Antignac C. Hum Mol Genet. 1997;6:2317–2323. - PubMed
-
- Sherman F E, Studnicki F M, Fetterman G. Am J Clin Pathol. 1971;55:391–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

