Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers
- PMID: 11493710
- PMCID: PMC55554
- DOI: 10.1073/pnas.171539698
Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers
Abstract
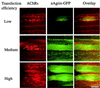
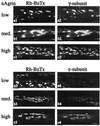
At mammalian neuromuscular junctions (NMJs), innervation induces and maintains the metabolic stability of acetylcholine receptors (AChRs). To explore whether neural agrin may cause similar receptor stabilization, we injected neural agrin cDNA of increasing transfection efficiencies into denervated adult rat soleus (SOL) muscles. As the efficiency increased, the amount of recombinant neural agrin expressed in the muscles also increased. This agrin aggregated AChRs on muscle fibers, whose half-life increased in a dose-dependent way from 1 to 10 days. Electrical muscle stimulation enhanced the stability of AChRs with short half-lives. Therefore, neural agrin can stabilize aggregated AChRs in a concentration- and activity-dependent way. However, there was no effect of stimulation on AChRs with a long half-life (10 days). Thus, at sufficiently high concentrations, neural agrin alone can stabilize AChRs to levels characteristic of innervated NMJs.
Figures



Similar articles
-
Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo.J Cell Biol. 2001 Jun 25;153(7):1441-52. doi: 10.1083/jcb.153.7.1441. J Cell Biol. 2001. PMID: 11425874 Free PMC article.
-
Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers.J Cell Biol. 2001 Jun 25;153(7):1453-63. doi: 10.1083/jcb.153.7.1453. J Cell Biol. 2001. PMID: 11425875 Free PMC article.
-
What controls the position, number, size, and distribution of neuromuscular junctions on rat muscle fibers?J Neurocytol. 2003 Jun-Sep;32(5-8):835-48. doi: 10.1023/B:NEUR.0000020627.18156.b1. J Neurocytol. 2003. PMID: 15034271 Review.
-
Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse.Dev Neurobiol. 2008 Aug;68(9):1153-69. doi: 10.1002/dneu.20654. Dev Neurobiol. 2008. PMID: 18506821
-
Neural agrin: a synaptic stabiliser.Int J Biochem Cell Biol. 2007;39(5):863-7. doi: 10.1016/j.biocel.2006.10.012. Epub 2006 Oct 25. Int J Biochem Cell Biol. 2007. PMID: 17126587 Review.
Cited by
-
Effects of delayed repair of peripheral nerve injury on the spatial distribution of motor endplates in target muscle.Neural Regen Res. 2022 Feb;17(2):459-464. doi: 10.4103/1673-5374.317990. Neural Regen Res. 2022. PMID: 34269223 Free PMC article.
-
Receptor tyrosine kinases in Drosophila development.Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a009050. doi: 10.1101/cshperspect.a009050. Cold Spring Harb Perspect Biol. 2013. PMID: 23732470 Free PMC article. Review.
-
Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves.Neural Regen Res. 2019 Apr;14(4):699-705. doi: 10.4103/1673-5374.247474. Neural Regen Res. 2019. PMID: 30632511 Free PMC article.
-
Agrin Influences Botulinum Neurotoxin A-Induced Nerve Sprouting via miR-144-agrin-MuSK Signaling.Front Cell Dev Biol. 2020 Jan 30;8:15. doi: 10.3389/fcell.2020.00015. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32083076 Free PMC article.
-
Non-synaptic roles of acetylcholinesterase and agrin.J Mol Neurosci. 2014 Jul;53(3):454-60. doi: 10.1007/s12031-013-0188-0. Epub 2013 Dec 11. J Mol Neurosci. 2014. PMID: 24326956
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

