Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone
- PMID: 11494116
- PMCID: PMC1505597
- DOI: 10.1038/sj.neo.7900151
Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone
Abstract
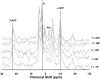
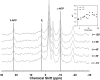
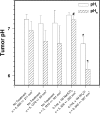
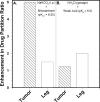
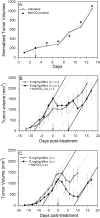
Uptake of weak acid and weak base chemotherapeutic drugs by tumors is greatly influenced by the tumor extracellular/interstitial pH (pH(e)), the intracellular pH (pH(i)) maintained by the tumor cells, and by the ionization properties of the drug itself. The acid-outside plasmalemmal pH gradient in tumors acts to exclude weak base drugs like the anthracyclines, anthraquinones, and vinca alkaloids from the cells, leading to a substantial degree of "physiological drug resistance" in tumors. We have induced acute metabolic alkalosis in C3H tumor-bearing C3H/hen mice, by gavage and by intraperitoneal (i.p.) administration of NaHCO(3). (31)P magnetic resonance spectroscopic measurements of 3-aminopropylphosphonate show increases of up to 0.6 pH units in tumor pH(e), and 0.2 to 0.3 pH units in hind leg tissue pH(e), within 2 hours of i.p. administration of NaHCO(3). Theoretical calculations of mitoxantrone uptake into tumor and normal (hind leg) tissue at the measured pH(e) and pH(i) values indicate that a gain in therapeutic index of up to 3.3-fold is possible with NaHCO(3) pretreatment. Treatment of C3H tumor-bearing mice with 12 mg/kg mitoxantrone resulted in a tumor growth delay of 9 days, whereas combined NaHCO(3)--mitoxantrone therapy resulted in an enhancement of the TGD to 16 days.
Figures
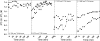

References
-
- Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. - PubMed
-
- Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential for exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. - PubMed
-
- Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2:343–366. - PubMed
-
- Gerweck LE. Tumor pH: implications for treatment and novel drug design. Semin Radiat Oncol. 1998;8:176–182. - PubMed
-
- Stubbs M, Bhujwalla ZM, Tozer GM, Rodrigues LM, Maxwell RJ, Morgan R, Howe FA, Griffiths JR. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992;5:351–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
