Modulation of L-type Ca2+ current but not activation of Ca2+ release by the gamma1 subunit of the dihydropyridine receptor of skeletal muscle
- PMID: 11495636
- PMCID: PMC37314
- DOI: 10.1186/1472-6793-1-8
Modulation of L-type Ca2+ current but not activation of Ca2+ release by the gamma1 subunit of the dihydropyridine receptor of skeletal muscle
Abstract
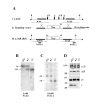
Background: The multisubunit (alpha1S,alpha2-delta, beta1a and gamma1) skeletal muscle dihydropyridine receptor (DHPR) transduces membrane depolarization into release of Ca2+ from the sarcoplasmic reticulum (SR) and also acts as an L-type Ca2+ channel. To more fully investigate the function of the gamma1 subunit in these two processes, we produced mice lacking this subunit by gene targeting.
Results: Mice lacking the DHPR gamma1 subunit (gamma1 null) survive to adulthood, are fertile and have no obvious gross phenotypic abnormalities. The gamma1 subunit is expressed at approximately half the normal level in heterozygous mice (gamma1 het). The density of the L-type Ca2+ current in gamma1 null and gamma1 het myotubes was higher than in controls. Inactivation of the Ca2+ current produced by a long depolarization was slower and incomplete in gamma1 null and gamma1 het myotubes, and was shifted to a more positive potential than in controls. However, the half-activation potential of intramembrane charge movements was not shifted, and the maximum density of the total charge was unchanged. Also, no shift was observed in the voltage-dependence of Ca2+ transients. gamma1 null and gamma1 het myotubes had the same peak Ca2+ amplitude vs. voltage relationship as control myotubes.
Conclusions: The L-type Ca2+ channel function, but not the SR Ca2+ release triggering function of the skeletal muscle dihydropyridine receptor, is modulated by the gamma1 subunit.
Figures



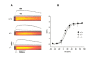
References
-
- Perez-Reyes E, Schneider T. Calcium channels: Structure, Function, and Classification. Drug Dev Res. 1994;33:295–318.
-
- McPherson PS, Campbell KP. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993;268:13765–13768. - PubMed
-
- Rios E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Revs. 1991;71:849–908. - PubMed
-
- Tanabe T, Beam KG, Adams BA, Niidome T, Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–572. - PubMed
-
- Nakai J, Tanabe T, Konno T, Adams B, Beam KG. Localization in the II-III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J Biol Chem. 1998;273:24983–24986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

