Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta
- PMID: 11495916
- PMCID: PMC1973163
- DOI: 10.1074/jbc.M105725200
Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta
Abstract
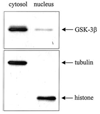
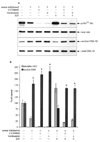
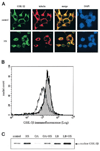
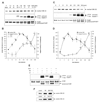
The goal of this study was to determine whether the intracellular distribution of the proapoptotic enzyme glycogen synthase kinase-3 beta (GSK-3 beta) is dynamically regulated by conditions that activate apoptotic signaling cascades. In untreated human neuroblastoma SH-SY5Y cells, GSK-3 beta was predominantly cytosolic, although a low level was also detected in the nucleus. The nuclear level of GSK-3 beta was rapidly increased after exposure of cells to serum-free media, heat shock, or staurosporine. Although each of these conditions caused changes in the serine 9 and/or tyrosine phosphorylation of GSK-3 beta, neither of these modifications was correlated with nuclear accumulation of GSK-3 beta. Heat shock and staurosporine treatments increased nuclear GSK-3 beta prior to activation of caspase-9 and caspase-3, and this nuclear accumulation of GSK-3 beta was unaltered by pretreatment with a general caspase inhibitor. The GSK-3 beta inhibitor lithium did not alter heat shock-induced nuclear accumulation of GSK-3 beta but increased the nuclear level of cyclin D1, indicating that cyclin D1 is a substrate of nuclear GSK-3 beta. Thus, the intracellular distribution of GSK-3 beta is dynamically regulated by signaling cascades, and apoptotic stimuli cause increased nuclear levels of GSK-3 beta, which facilitates interactions with nuclear substrates.
Figures



References
-
- Grimes CA, Jope RS. Prog. Neurobiol. 2001;65:391–426. - PubMed
-
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. J. Biol. Chem. 1994;269:14566–14574. - PubMed
-
- Lesort M, Jope RS, Johnson GV. J. Neurochem. 1999;72:576–584. - PubMed
-
- Hartigan JA, Johnson GV. J. Biol. Chem. 1999;274:21395–21401. - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Curr. Biol. 1997;7:261–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

