Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues
- PMID: 11498511
- PMCID: PMC1621149
- DOI: 10.1038/sj.bjp.0704197
Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues
Abstract
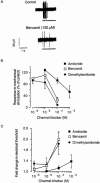
1. The aim of this study was to investigate a role for Epithelial Sodium Channels (ENaCs) in the mechanical activation of low-threshold vagal afferent nerve terminals in the guinea-pig trachea/bronchus. 2. Using extracellular single-unit recording techniques, we found that the ENaC blocker amiloride, and its analogues dimethylamiloride and benzamil caused a reduction in the mechanical activation of guinea-pig airway afferent fibres. 3. Amiloride and it analogues also reduced the sensitivity of afferent fibres to electrical stimulation such that greater stimulation voltages were required to induce action potentials from their peripheral terminals within the trachea/bronchus. 4. The relative potencies of these compounds for inhibiting electrical excitability of afferent nerves were similar to that observed for inhibition of mechanical stimulation (dimethylamiloride approximately benzamil > amiloride). This rank order of potency is incompatible with the known rank order of potency for blockade of ENaCs (benzamil > amiloride >> dimethylamiloride). 5. As voltage-gated sodium channels play an important role in determining the electrical excitability of neurons, we used whole-cell patch recordings of nodose neuron cell bodies to investigate the possibility that amiloride analogues caused blockade of these channels. At the concentration required to inhibit mechanical activation of vagal nodose afferent fibres (100 microM), benzamil caused significant inhibition of voltage-gated sodium currents in neuronal cell bodies acutely isolated from guinea-pig nodose ganglia. 6. Combined, our findings suggest that amiloride and its analogues did not selectively block mechanotransduction in airway afferent neurons, but rather they reduced neuronal excitability, possibly by inhibiting voltage-gated sodium currents.
Figures


References
-
- ALVAREZ DE LA ROSA D., CANESSA C.M., FYFE G.K., ZHANG P. Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 2000;62:573–594. - PubMed
-
- ANDRESEN M.C., YANG M. Gadolinium and mechanotransduction of rat aortic baroreceptors. Am. J. Physiol. 1992;262:H1415–H1421. - PubMed
-
- AWAYDA M.S., ISMAILOV I.I., BERDIEV B.K., BENOS D.J. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am. J. Physiol. 1995;268:C1450–C1459. - PubMed
-
- BROUNS I., ADRIAENSEN D., BURNSTOCK G., TIMMERMANS J.P. Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X(3) receptors. Am. J. Respir. Cell Mol. Biol. 2000;23:52–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

