New EMBO members' review: viral and bacterial proteins regulating apoptosis at the mitochondrial level
- PMID: 11500358
- PMCID: PMC125565
- DOI: 10.1093/emboj/20.16.4325
New EMBO members' review: viral and bacterial proteins regulating apoptosis at the mitochondrial level
Abstract
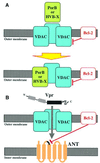
Mitochondrial membrane permeabilization (MMP) is a critical step of several apoptotic pathways. Some infectious intracellular pathogens can regulate (induce or inhibit) apoptosis of their host cells at the mitochondrial level, by targeting proteins to mitochondrial membranes that either induce or inhibit MMP. Pathogen-encoded mitochondrion-targeted proteins may or may not show amino acid sequence homology to Bcl-2-like proteins. Among the Bcl-2-unrelated, mitochondrion-targeted proteins, several interact with the voltage-dependent anion channel (VDAC) or with the adenine nucleotide translocator (ANT). While VDAC-targeted proteins show homology to VDAC/porin, ANT-targeted proteins possess relatively short cationic binding domains, which may facilitate insertion into the negatively charged inner mitochondrial membrane. It may be speculated that such proteins employ pre-existing host-intrinsic mechanisms of MMP control.
Figures

References
-
- Brenner C. et al. (2000) Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene, 19, 329–336. - PubMed
-
- Ciminale V., Zotti,L., D’Agostini,D.M., Ferro,T., Casareto,L., Franchini,G., Bernardi,P. and Chieco Bianchi,L. (1999) Mitochondrial targeting of the p13(II) protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-1). Oncogene, 18, 4505–4514. - PubMed
-
- Colberg-Poley A.M., Patel,M.B., Erezo,D.P. and Slater,J.E. (2000) Human cytomegalovirus UL37 immediate-early regulatory proteins traffic through the secretory apparatus and to mitochondria. J. Gen. Virol., 81, 1779–1789. - PubMed
-
- Cowan S.W., Schirmer,T., Rummel,G., Steier,M., Gosh,R., Pauptit,R.A., Jansonius,J.N. and Rosenbusch,J.P. (1992) Crystal structures explain functional properties of two E.coli porins. Nature, 358, 727–733. - PubMed
-
- De Laurenzi V. and Melino,G. (2000) Apoptosis. The little devil of death. Nature, 406, 135–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

