Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase
- PMID: 11500364
- PMCID: PMC125559
- DOI: 10.1093/emboj/20.16.4370
Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase
Abstract
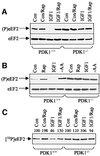
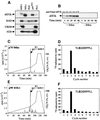
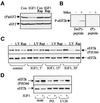
Elongation factor 2 kinase (eEF2k) phosphorylates and inactivates eEF2. Insulin induces dephosphorylation of eEF2 and inactivation of eEF2 kinase, and these effects are blocked by rapamycin, which inhibits the mammalian target of rapamycin, mTOR. However, the signalling mechanisms underlying these effects are unknown. Regulation of eEF2 phosphorylation and eEF2k activity is lost in cells in which phosphoinositide-dependent kinase 1 (PDK1) has been genetically knocked out. This is not due to loss of mTOR function since phosphorylation of another target of mTOR, initiation factor 4E-binding protein 1, is not defective. PDK1 is required for activation of members of the AGC kinase family; we show that two such kinases, p70 S6 kinase (regulated via mTOR) and p90(RSK1) (activated by Erk), phosphorylate eEF2k at a conserved serine and inhibit its activity. In response to insulin-like growth factor 1, which activates p70 S6 kinase but not Erk, regulation of eEF2 is blocked by rapamycin. In contrast, regulation of eEF2 by stimuli that activate Erk is insensitive to rapamycin, but blocked by inhibitors of MEK/Erk signalling, consistent with the involvement of p90(RSK1).
Figures





References
-
- Alessi D.R., Kozlowski,M.T., Weng,Q.-P., Morrice,N. and Avruch,J. (1998) 3-phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol., 8, 69–81. - PubMed
-
- Balendran A., Currie,R., Armstrong,C.G., Avruch,J. and Alessi,D.R. (1999) Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J. Biol. Chem., 274, 37400–37406. - PubMed
-
- Brunn G.J., Hudson,C.C., Sekulic,A., Williams,J.M., Hosoi,H., Houghton,P.J., Lawrence,J.C. and Abraham,R.T. (1997) Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science, 277, 99–101. - PubMed
-
- Burgering B.M.T. and Coffer,P.J. (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature, 376, 599–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

