Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain
- PMID: 11500373
- PMCID: PMC125583
- DOI: 10.1093/emboj/20.16.4454
Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain
Abstract
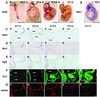
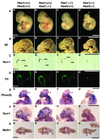
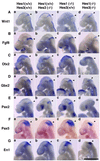
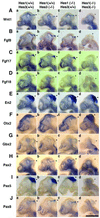
The isthmic organizer, which is located at the midbrain-hindbrain boundary, plays an essential role in development of the midbrain and anterior hindbrain. It has been shown that homeobox genes regulate establishment of the isthmic organizer, but the mechanism by which the organizer is maintained is not well understood. Here, we found that, in mice doubly mutant for the basic helix-loop-helix genes Hes1 and Hes3, the midbrain and anterior hindbrain structures are missing without any significant cell death. In these mutants, the isthmic organizer cells prematurely differentiate into neurons and terminate expression of secreting molecules such as Fgf8 and Wnt1 and the paired box genes Pax2/5, all of which are essential for the isthmic organizer function. These results indicate that Hes1 and Hes3 prevent premature differentiation and maintain the organizer activity of the isthmic cells, thereby regulating the development of the midbrain and anterior hindbrain.
Figures




References
-
- Akazawa C., Ishibashi,M., Shimizu,C., Nakanishi,S. and Kageyama,R. (1995) A mammalian helix–loop–helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J. Biol. Chem., 270, 8730–8738. - PubMed
-
- Allen T. and Lobe,C.G. (1999) A comparison of Notch, Hes and Grg expression during murine embryonic and post-natal development. Cell. Mol. Biol., 45, 687–708. - PubMed
-
- Bally-Cuif L., Cholley,B. and Wassef,M. (1995) Involvement of Wnt1 in the formation of the mes/metencephalic boundary. Mech. Dev., 53, 23–34. - PubMed
-
- Beddington R.S.P. and Robertson,E.J. (1998) Anterior patterning in mouse. Trends Genet., 14, 277–284. - PubMed
-
- Ben-Arie N., Hassan,B.A., Bermingham,N.A., Malicki,D.M., Armstrong,D., Matzuk,M., Bellen,H.J. and Zoghbi,H.Y. (2000) Functional conservation of atonal and Math1 in the CNS and PNS. Development, 127, 1039–1048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

