Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems
- PMID: 11500438
- PMCID: PMC98678
- DOI: 10.1128/IAI.69.9.5626-5634.2001
Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems
Abstract
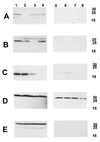
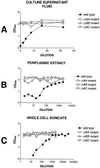
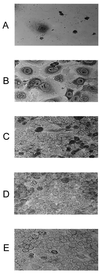
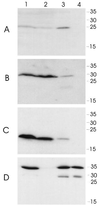
Haemophilus ducreyi expresses a soluble cytolethal distending toxin (CDT) that is encoded by the cdtABC gene cluster and can be detected in culture supernatant fluid by its ability to kill HeLa cells. The cdtA, cdtB, and cdtC genes of H. ducreyi were cloned independently into plasmid vectors, and their encoded proteins expressed singly or in various combinations in an Escherichia coli background. All three gene products had to be expressed in order for E. coli-derived culture supernatant fluids to demonstrate cytotoxicity for HeLa cells. Isogenic H. ducreyi cdtA and cdtB mutants were constructed and used in combination with the wild-type parent strain and a previously described H. ducreyi cdtC mutant (M. K. Stevens, J. L. Latimer, S. R. Lumbley, C. K. Ward, L. D. Cope, T. Lagergard, and E. J. Hansen, Infect. Immun. 67:3900-3908, 1999) to determine the relative contributions of the CdtA, CdtB, and CdtC proteins to CDT activity. Expression of CdtA, CdtB, and CdtC appeared necessary for H. ducreyi-derived culture supernatant fluid to exhibit cytotoxicity for HeLa cells. Whole-cell sonicates and periplasmic extracts from the cdtB and cdtC mutants had no effect on HeLa cells, whereas these same fractions from a cdtA mutant had a very modest cytotoxic effect on these same human cells. CdtA appeared to be primarily associated with the H. ducreyi cell envelope, whereas both CdtB and CdtC were present primarily in the soluble fraction from sonicated cells. Both the cdtA mutant and the cdtB mutant were found to be fully virulent in the temperature-dependent rabbit model for experimental chancroid.
Figures

Similar articles
-
Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin.Infect Immun. 1999 Aug;67(8):3900-8. doi: 10.1128/IAI.67.8.3900-3908.1999. Infect Immun. 1999. PMID: 10417154 Free PMC article.
-
A CdtA-CdtC complex can block killing of HeLa cells by Haemophilus ducreyi cytolethal distending toxin.Infect Immun. 2003 Nov;71(11):6633-40. doi: 10.1128/IAI.71.11.6633-6640.2003. Infect Immun. 2003. PMID: 14573688 Free PMC article.
-
Investigation of the interaction among the components of the cytolethal distending toxin of Haemophilus ducreyi.Biochem Biophys Res Commun. 2001 Jul 20;285(3):609-15. doi: 10.1006/bbrc.2001.5223. Biochem Biophys Res Commun. 2001. PMID: 11453636
-
Cytolethal distending toxins and activation of DNA damage-dependent checkpoint responses.Int J Med Microbiol. 2002 Feb;291(6-7):495-9. doi: 10.1078/1438-4221-00158. Int J Med Microbiol. 2002. PMID: 11890549 Review.
-
In vivo virulence properties of bacterial cytolethal-distending toxin.Cell Microbiol. 2008 Aug;10(8):1599-607. doi: 10.1111/j.1462-5822.2008.01173.x. Epub 2008 May 16. Cell Microbiol. 2008. PMID: 18489725 Review.
Cited by
-
Studying the Interaction of Neutrophils and Glaesserella Parasuis Indicates a Serotype Independent Benefit from Degradation of NETs.Pathogens. 2022 Aug 4;11(8):880. doi: 10.3390/pathogens11080880. Pathogens. 2022. PMID: 36015001 Free PMC article.
-
The Enterobacterial Genotoxins: Cytolethal Distending Toxin and Colibactin.EcoSal Plus. 2016 Jul;7(1):10.1128/ecosalplus.ESP-0008-2016. doi: 10.1128/ecosalplus.ESP-0008-2016. EcoSal Plus. 2016. PMID: 27419387 Free PMC article. Review.
-
Differential expression of porins OmpP2A and OmpP2B of Haemophilus ducreyi.Infect Immun. 2004 Nov;72(11):6271-8. doi: 10.1128/IAI.72.11.6271-6278.2004. Infect Immun. 2004. PMID: 15501753 Free PMC article.
-
Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages.Microbiology (Reading). 2011 Jul;157(Pt 7):1851-1875. doi: 10.1099/mic.0.049536-0. Epub 2011 May 12. Microbiology (Reading). 2011. PMID: 21565933 Free PMC article. Review.
-
Mechanisms of assembly and cellular interactions for the bacterial genotoxin CDT.PLoS Pathog. 2005 Nov;1(3):e28. doi: 10.1371/journal.ppat.0010028. Epub 2005 Nov 18. PLoS Pathog. 2005. PMID: 16304609 Free PMC article.
References
-
- Al-Tawfiq J A, Palmer K L, Chen C-Y, Haley J C, Katz B P, Hood A F, Spinola S M. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J Infect Dis. 1999;179:1283–1287. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

