The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein
- PMID: 11502763
- PMCID: PMC2196466
- DOI: 10.1083/jcb.200104045
The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein
Abstract
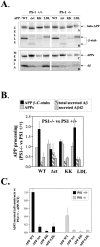
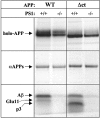
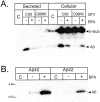
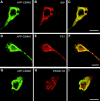
We investigated the relationship between PS1 and gamma-secretase processing of amyloid precursor protein (APP) in primary cultures of neurons. Increasing the amount of APP at the cell surface or towards endosomes did not significantly affect PS1-dependent gamma-secretase cleavage, although little PS1 is present in those subcellular compartments. In contrast, almost no gamma-secretase processing was observed when holo-APP or APP-C99, a direct substrate for gamma-secretase, were specifically retained in the endoplasmic reticulum (ER) by a double lysine retention motif. Nevertheless, APP-C99-dilysine (KK) colocalized with PS1 in the ER. In contrast, APP-C99 did not colocalize with PS1, but was efficiently processed by PS1-dependent gamma-secretase. APP-C99 resides in a compartment that is negative for ER, intermediate compartment, and Golgi marker proteins. We conclude that gamma-secretase cleavage of APP-C99 occurs in a specialized subcellular compartment where little or no PS1 is detected. This suggests that at least one other factor than PS1, located downstream of the ER, is required for the gamma-cleavage of APP-C99. In agreement, we found that intracellular gamma-secretase processing of APP-C99-KK both at the gamma40 and the gamma42 site could be restored partially after brefeldin A treatment. Our data confirm the "spatial paradox" and raise several questions regarding the PS1 is gamma-secretase hypothesis.
Figures


References
-
- Annaert, W., and B. De Strooper. 1999. Presenilins: molecular switches between proteolysis and signal transduction. Trends Neurosci. 22:439–443. - PubMed
-
- Annaert, W.G., L. Levesque, K. Craessaerts, I. Dierinck, G. Snellings, D. Westaway, P.S. George-Hyslop, B. Cordell, P. Fraser, and B. De Strooper. 1999. Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J. Cell Biol. 147:277–294. - PMC - PubMed
-
- Barelli, H., A. Lebeau, J. Vizzanova, P. Delaere, N. Chevalier, C. Drouot, P. Marambaud, K. Ancolio, J.D. Buxbaum, O. Khorkova, et al. 1997. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid long amyloid β peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol. Med. 3: 695–707. - PMC - PubMed
-
- Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J.R. Doedens, A. Cumano, P. Roux, R.A. Black, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 5:207–216. - PubMed
-
- Brown, M.S., J. Ye, R.B. Rawson, and J.L. Goldstein. 2000. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 100:391–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

