Thermodynamic stability of base pairs between 2-hydroxyadenine and incoming nucleotides as a determinant of nucleotide incorporation specificity during replication
- PMID: 11504865
- PMCID: PMC55858
- DOI: 10.1093/nar/29.16.3289
Thermodynamic stability of base pairs between 2-hydroxyadenine and incoming nucleotides as a determinant of nucleotide incorporation specificity during replication
Abstract
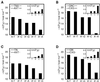
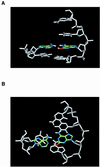
We investigated the thermodynamic stability of double-stranded DNAs with an oxidative DNA lesion, 2-hydroxyadenine (2-OH-Ade), in two different sequence contexts (5'-GA*C-3' and 5'-TA*A-3', A* represents 2-OH-Ade). When an A*-N pair (N, any nucleotide base) was located in the center of a duplex, the thermodynamic stabilities of the duplexes were similar for all the natural bases except A (N = T, C and G). On the other hand, for the duplexes with the A*-N pair at the end, which mimic the nucleotide incorporation step, the stabilities of the duplexes were dependent on their sequence. The order of stability is T > G > C >> A in the 5'-GA*C-3' sequences and T > A > C > G in the 5'-TA*A-3' sequences. Because T/G/C and T/A are nucleotides incorporated opposite to 2-OH-Ade in the 5'-GA*C-3' and 5'-TA*A-3' sequences, respectively, these results agree with the tendency of mutagenic misincorporation of the nucleotides opposite to 2-OH-Ade in vitro. Thus, the thermodynamic stability of the A*-N base pair may be an important factor for the mutation spectra of 2-OH-Ade.
Figures

Similar articles
-
Effect of sequence contexts on misincorporation of nucleotides opposite 2-hydroxyadenine.FEBS Lett. 1996 Aug 5;391(1-2):113-6. doi: 10.1016/0014-5793(96)00714-4. FEBS Lett. 1996. PMID: 8706896
-
DNA sequence context modulates the impact of a cisplatin 1,2-d(GpG) intrastrand cross-link on the conformational and thermodynamic properties of duplex DNA.J Mol Biol. 2000 Feb 25;296(3):803-12. doi: 10.1006/jmbi.2000.3496. J Mol Biol. 2000. PMID: 10677282
-
Evading the proofreading machinery of a replicative DNA polymerase: induction of a mutation by an environmental carcinogen.J Mol Biol. 2001 Jun 1;309(2):519-36. doi: 10.1006/jmbi.2001.4674. J Mol Biol. 2001. PMID: 11371169
-
Mutagenicities of 8-hydroxyguanine and 2-hydroxyadenine produced by reactive oxygen species.Biol Pharm Bull. 2004 Apr;27(4):475-9. doi: 10.1248/bpb.27.475. Biol Pharm Bull. 2004. PMID: 15056850 Review.
-
Survey and summary: The applications of universal DNA base analogues.Nucleic Acids Res. 2001 Jun 15;29(12):2437-47. doi: 10.1093/nar/29.12.2437. Nucleic Acids Res. 2001. PMID: 11410649 Free PMC article. Review.
Cited by
-
Error-free bypass of 2-hydroxyadenine by human DNA polymerase lambda with Proliferating Cell Nuclear Antigen and Replication Protein A in different sequence contexts.Nucleic Acids Res. 2007;35(15):5173-81. doi: 10.1093/nar/gkm568. Epub 2007 Jul 30. Nucleic Acids Res. 2007. PMID: 17666409 Free PMC article.
-
Neighbouring bases in template influence base-pairing of isoguanine.Biochem J. 2003 Feb 1;369(Pt 3):611-8. doi: 10.1042/BJ20020922. Biochem J. 2003. PMID: 12387728 Free PMC article.
-
Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary.Nucleic Acids Res. 2003 Jan 15;31(2):517-31. doi: 10.1093/nar/gkg137. Nucleic Acids Res. 2003. PMID: 12527759 Free PMC article. Review.
-
Replication of 2-hydroxyadenine-containing DNA and recognition by human MutSalpha.DNA Repair (Amst). 2007 Mar 1;6(3):355-66. doi: 10.1016/j.dnarep.2006.11.002. Epub 2006 Dec 26. DNA Repair (Amst). 2007. PMID: 17188944 Free PMC article.
References
-
- Wallace S.S. (1994) DNA damages processed by base excision repair: biological consequences. Int. J. Radiat. Biol., 66, 579–589. - PubMed
-
- Kasai H. and Nishimura,S. (1991) Formation of 8-hydroxydeoxyguanosine in DNA by oxygen radicals and its biological significance. In Sies,H. (ed.), Oxidative Stress: Oxidants and Antioxidants. Academic Press, San Diego, CA, pp. 99–116.
-
- Kamiya H., Miura,K., Ishikawa,H., Inoue,H., Nishimura,S. and Ohtsuka,E. (1992) c-Ha-ras containing 8-hydroxyguanine at codon 12 induces point mutations at the modified and adjacent positions. Cancer Res., 52, 3483–3485. - PubMed
-
- Kamiya H., Murata-Kamiya,N., Koizume,S., Inoue,H., Nishimura,S. and Ohtsuka,E. (1995) 8-Hydroxyguanine (7,8-dihydro-8-oxoguanine) in hot spots of the c-Ha-ras gene: effects of sequence contexts on mutation spectra. Carcinogenesis, 16, 883–889. - PubMed
-
- Wagner J., Kamiya,H. and Fuchs,R.P. (1997) Leading versus lagging strand mutagenesis induced by 7,8-dihydro-8-oxo-2′-deoxyguanosine in Escherichia coli. J. Mol. Biol., 265, 302–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

