Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA
- PMID: 11504881
- PMCID: PMC55850
- DOI: 10.1093/nar/29.16.3433
Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA
Abstract
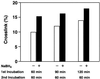
Methylglyoxal, a known endogenous and environmental mutagen, is a reactive alpha-ketoaldehyde that can modify both DNA and proteins. To investigate the possibility that methylglyoxal induces a crosslink between DNA and DNA polymerase, we treated a 'primed template' DNA and the exonuclease-deficient Klenow fragment (KF(exo-)) of DNA polymerase I with methylglyoxal in vitro. When the reaction mixtures were analyzed by SDS-PAGE, we found that methylglyoxal induced a DNA-KF(exo-) crosslink. The specific binding complex of KF(exo-) and 'primed template' DNA was necessary for formation of the DNA-KF(exo-) crosslink. Methylglyoxal reacted with guanine residues in the single-stranded portion of the template DNA. When 2'-deoxyguanosine was incubated with Nalpha-acetyllysine or N-acetylcysteine in the presence of methylglyoxal, a crosslinked product was formed. No other amino acid derivatives tested could generate a crosslinked product. These results suggest that methylglyoxal crosslinks a guanine residue of the substrate DNA and lysine and cysteine residues near the binding site of the DNA polymerase during DNA synthesis and that DNA replication is severely inhibited by the methylglyoxal-induced DNA-DNA polymerase crosslink.
Figures





References
-
- Richard J.P. (1993) Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans., 21, 549–553. - PubMed
-
- Phillips S.A and Thornalley,P.J. (1993) The formation of methylglyoxal from triose phosphates: investigation using a specific assay for methylglyoxal. Eur. J. Biochem., 212, 101–105. - PubMed
-
- Poli G., Dianzani,M.U., Cheeseman,K.H., Slater,T.F., Lang,J. and Esterbauer,H. (1985) Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem. J., 227, 629–638. - PMC - PubMed
-
- Reichard G.A.,Jr, Skutches,C.L., Hoeldtke,R.D. and Owen,O.E. (1986) Acetone metabolism in humans during diabetic ketoacidosis. Diabetes, 35, 668–674. - PubMed
-
- Lyles G.A. and Chalmers,J. (1992) The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem. Pharmacol., 43, 1409–1414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

