Mobilized bone marrow cells repair the infarcted heart, improving function and survival
- PMID: 11504914
- PMCID: PMC56963
- DOI: 10.1073/pnas.181177898
Mobilized bone marrow cells repair the infarcted heart, improving function and survival
Abstract
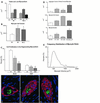
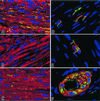
Attempts to repair myocardial infarcts by transplanting cardiomyocytes or skeletal myoblasts have failed to reconstitute healthy myocardium and coronary vessels integrated structurally and functionally with the remaining viable portion of the ventricular wall. The recently discovered growth and transdifferentiation potential of primitive bone marrow cells (BMC) prompted us, in an earlier study, to inject in the border zone of acute infarcts Lin(-) c-kit(POS) BMC from syngeneic animals. These BMC differentiated into myocytes and vascular structures, ameliorating the function of the infarcted heart. Two critical determinants seem to be required for the transdifferentiation of primitive BMC: tissue damage and a high level of pluripotent cells. On this basis, we hypothesized here that BMC, mobilized by stem cell factor and granulocyte-colony stimulating factor, would home to the infarcted region, replicate, differentiate, and ultimately promote myocardial repair. We report that, in the presence of an acute myocardial infarct, cytokine-mediated translocation of BMC resulted in a significant degree of tissue regeneration 27 days later. Cytokine-induced cardiac repair decreased mortality by 68%, infarct size by 40%, cavitary dilation by 26%, and diastolic stress by 70%. Ejection fraction progressively increased and hemodynamics significantly improved as a consequence of the formation of 15 x 10(6) new myocytes with arterioles and capillaries connected with the circulation of the unaffected ventricle. In conclusion, mobilization of primitive BMC by cytokines might offer a noninvasive therapeutic strategy for the regeneration of the myocardium lost as a result of ischemic heart disease and, perhaps, other forms of cardiac pathology.
Figures


References
-
- Beltrami A, Urbanek K, Kajstura J, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami C A, Anversa P. N Engl J Med. 2001;344:1750–1757. - PubMed
-
- Linzbach A J. Am J Cardiol. 1960;5:370–382. - PubMed
-
- Leor J, Patterson M, Quinones M J, Kedes L H, Kloner R A. Circulation. 1996;94,Suppl.:II331–II336. - PubMed
-
- Taylor D A, Atkins B Z, Hungspreugs P, Jones T R, Reedy M C, Hutcheson K A, Glower D D, Kraus W E. Nat Med. 1998;4:929–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

