Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease
- PMID: 11504916
- PMCID: PMC56978
- DOI: 10.1073/pnas.181182298
Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease
Abstract
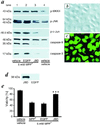
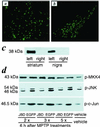
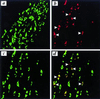
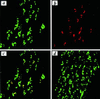
Increasing evidence suggests that apoptosis may be the underlying cell death mechanism in the selective loss of dopaminergic neurons in Parkinson's disease. Because the inhibition of caspases provides only partial protection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/1-methyl-4-phenylpyridinium (MPTP/MPP(+)) model of Parkinson's disease, we investigated the role of the proapoptotic c-Jun N-terminal kinase (JNK) signaling cascade in SH-SY5Y human neuroblastoma cells in vitro and in mice in vivo. MPTP/MPP(+) led to the sequential phosphorylation and activation of JNK kinase (MKK4), JNK, and c-Jun, the activation of caspases, and apoptosis. In mice, adenoviral gene transfer of the JNK binding domain of JNK-interacting protein-1 (a scaffold protein and inhibitor of JNK) inhibited this cascade downstream of MKK4 phosphorylation, blocked JNK, c-Jun, and caspase activation, the death of dopaminergic neurons, and the loss of catecholamines in the striatum. Furthermore, the gene transfer resulted in behavioral benefit. Therefore, inhibition of the JNK pathway offers a new treatment strategy for Parkinson's disease that blocks the death signaling pathway upstream of the execution of apoptosis in dopaminergic neurons, providing a therapeutic advantage over the direct inhibition of caspases.
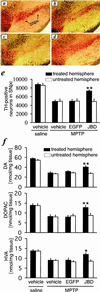
Figures
References
-
- Zhang Y, Dawson V L, Dawson T M. Neurobiol Dis. 2000;7:240–250. - PubMed
-
- de Silva H R, Khan N L, Wood N W. Curr Opin Genet Dev. 2000;10:292–298. - PubMed
-
- Beal M F. Ann Neurol. 1995;38:357–366. - PubMed
-
- Jenner P, Olanow C W. Ann Neurol. 1998;44:S72–S84. - PubMed
-
- Honig L S, Rosenberg R N. Am J Med. 2000;108:317–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

