Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion
- PMID: 11504920
- PMCID: PMC56979
- DOI: 10.1073/pnas.181219298
Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion
Abstract
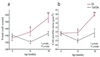
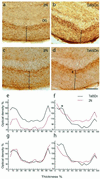
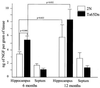
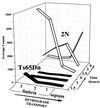
Age-related degeneration of basal forebrain cholinergic neurons (BFCNs) contributes to cognitive decline in Alzheimer's disease and Down's syndrome. With aging, the partial trisomy 16 (Ts65Dn) mouse model of Down's syndrome exhibited reductions in BFCN size and number and regressive changes in the hippocampal terminal fields of these neurons with respect to diploid controls. The changes were associated with significantly impaired retrograde transport of nerve growth factor (NGF) from the hippocampus to the basal forebrain. Intracerebroventricular NGF infusion reversed well established abnormalities in BFCN size and number and restored the deficit in cholinergic innervation. The findings are evidence that even BFCNs chronically deprived of endogenous NGF respond to an intervention that compensates for defective retrograde transport. We suggest that age-related cholinergic neurodegeneration may be a treatable disorder of failed retrograde NGF signaling.
Figures
Similar articles
-
Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration.Neuron. 2006 Jul 6;51(1):29-42. doi: 10.1016/j.neuron.2006.05.022. Neuron. 2006. PMID: 16815330
-
Nerve growth factor metabolic dysfunction in Down's syndrome brains.Brain. 2014 Mar;137(Pt 3):860-72. doi: 10.1093/brain/awt372. Epub 2014 Feb 11. Brain. 2014. PMID: 24519975 Free PMC article.
-
Minocycline prevents cholinergic loss in a mouse model of Down's syndrome.Ann Neurol. 2004 Nov;56(5):675-88. doi: 10.1002/ana.20250. Ann Neurol. 2004. PMID: 15468085
-
Alzheimer's disease and NGF signaling.J Neural Transm (Vienna). 2004 Mar;111(3):323-45. doi: 10.1007/s00702-003-0091-x. J Neural Transm (Vienna). 2004. PMID: 14991458 Review.
-
The cholinergic system in aging and neuronal degeneration.Behav Brain Res. 2011 Aug 10;221(2):555-63. doi: 10.1016/j.bbr.2010.11.058. Epub 2010 Dec 9. Behav Brain Res. 2011. PMID: 21145918 Review.
Cited by
-
The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a006361. doi: 10.1101/cshperspect.a006361. Cold Spring Harb Perspect Med. 2012. PMID: 22908190 Free PMC article.
-
The power of comparative and developmental studies for mouse models of Down syndrome.Mamm Genome. 2007 Jul;18(6-7):431-43. doi: 10.1007/s00335-007-9030-8. Epub 2007 Jul 26. Mamm Genome. 2007. PMID: 17653795 Free PMC article. Review.
-
A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions.Hum Mol Genet. 2010 Jul 15;19(14):2780-91. doi: 10.1093/hmg/ddq179. Epub 2010 May 4. Hum Mol Genet. 2010. PMID: 20442137 Free PMC article.
-
Longitudinal manganese-enhanced magnetic resonance imaging of neural projections and activity.NMR Biomed. 2022 Jun;35(6):e4675. doi: 10.1002/nbm.4675. Epub 2022 Mar 6. NMR Biomed. 2022. PMID: 35253280 Free PMC article. Review.
-
Cognitive deficit associated with cholinergic and nerve growth factor down-regulation in experimental allergic encephalomyelitis in rats.Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):3070-5. doi: 10.1073/pnas.0500073102. Epub 2005 Feb 14. Proc Natl Acad Sci U S A. 2005. PMID: 15710875 Free PMC article.
References
-
- Whitehouse P J, Price D L, Struble R G, Clark A W, Coyle J T, Delong M R. Science. 1982;215:1237–1239. - PubMed
-
- Mufson E J, Bothwell M, Kordower J H. Exp Neurol. 1989;105:221–232. - PubMed
-
- Casanova M F, Walker L C, Whitehouse P J, Price D L. Ann Neurol. 1985;18:310–313. - PubMed
-
- Mann D M, Yates P O, Marcyniuk B. Neuropathol Appl Neurobiol. 1984;10:185–207. - PubMed
-
- Reeves R H, Irving N G, Moran T H, Wohn A, Kitt C, Sisodia S S, Schmidt C, Bronson R T, Davisson M T. Nat Genet. 1995;11:177–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

