Self-organization of a radial microtubule array by dynein-dependent nucleation of microtubules
- PMID: 11504928
- PMCID: PMC56932
- DOI: 10.1073/pnas.181354198
Self-organization of a radial microtubule array by dynein-dependent nucleation of microtubules
Abstract
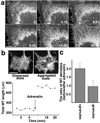
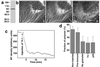
Polarized radial arrays of cytoplasmic microtubules (MTs) with minus ends clustered at the cell center define the organization of the cytoplasm through interaction with microtubule motors bound to membrane organelles or chromosomes. It is generally assumed that the radial organization results from nucleation of MTs at the centrosome. However, radial MT array can also be attained through self-organization that requires the activity of a minus-end-directed MT motor, cytoplasmic dynein. In this study we examine the role of cytoplasmic dynein in the self-organization of a radial MT array in cytoplasmic fragments of fish melanophores lacking the centrosome. After activation of dynein motors bound to membrane-bound organelles, pigment granules, the fragments rapidly form polarized radial arrays of MTs and position pigment aggregates at their centers. We show that rearrangement of MTs in the cytoplasm is achieved through dynein-dependent MT nucleation. The radial pattern is generated by continuous disassembly and reassembly of MTs and concurrent minus-end-directed transport of pigment granules bearing the nucleation sites.
Figures



References
-
- Kellog D R, Moritz M, Alberts B. Annu Rev Biochem. 1994;63:639–674. - PubMed
-
- Mitchison T J, Kirschner M A. Nature (London) 1984;312:237–242. - PubMed
-
- Lane J, Allan V. Biochim Biophys Acta. 1998;1376:27–55. - PubMed
-
- Goldstein L S, Philp A V. Annu Rev Cell Dev Biol. 1999;15:141–183. - PubMed
-
- Holzbaur E, Vallee R B. Annu Rev Cell Biol. 1994;10:331–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

