Characterization of an adapter subunit to a phosphatidylinositol (3)P 3-phosphatase: identification of a myotubularin-related protein lacking catalytic activity
- PMID: 11504939
- PMCID: PMC55481
- DOI: 10.1073/pnas.171306098
Characterization of an adapter subunit to a phosphatidylinositol (3)P 3-phosphatase: identification of a myotubularin-related protein lacking catalytic activity
Abstract
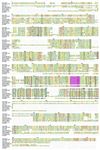
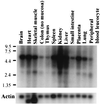
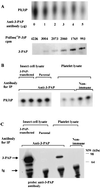
The D3-phosphoinositides act as second messengers by recruiting, and thereby activating, diverse signaling proteins. We have previously described the purification of a rat phosphatidylinositol 3-phosphate [PtdIns(3)P] 3-phosphatase, comprising a heterodimer of a 78-kDa adapter subunit in complex with a 65-kDa catalytic subunit. Here, we have cloned and characterized the cDNA encoding the human 3-phosphatase adapter subunit (3-PAP). Sequence alignment showed that 3-PAP shares significant sequence similarity with the protein and lipid 3-phosphatase myotubularin, and with several other members of the myotubularin gene family including SET-binding factor 1. However, unlike myotubularin, 3-PAP does not contain a consensus HCX(5)R catalytic motif. The 3-PAP sequence contains several motifs that predict interaction with proteins containing Src homology-2 (SH2) domains, phosphotyrosine-binding (PTB) domains, members of the 14-3-3 family, as well as proteins with SET domains. Northern blot analysis identified two transcripts (5.5 kb and 2.5 kb) with highest abundance in human liver, kidney, lung, and placenta. 3-PAP immunoprecipitates isolated from platelet cytosol hydrolyzed the D3-phosphate from PtdIns(3)P and PtdIns 3,4-bisphosphate [PtdIns(3,4)P(2)]. However, insect cell-expressed 3-PAP recombinant protein was catalytically inactive, confirming our prior prediction that this polypeptide represents an adapter subunit.
Figures


References
-
- Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Trends Biochem Sci. 1997;22:267–272. - PubMed
-
- Rameh L E, Cantley L C. J Biol Chem. 1999;274:8347–8350. - PubMed
-
- Czech M P. Cell. 2000;100:603–606. - PubMed
-
- Datta S R, Brunet A, Greenberg M E. Genes Dev. 1999;13:2905–2927. - PubMed
-
- Corvera S, D'Arrigo A, Stenmark H. Curr Opin Cell Biol. 1999;11:460–465. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

