Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels
- PMID: 11507158
- PMCID: PMC2278757
- DOI: 10.1111/j.1469-7793.2001.00065.x
Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels
Abstract
1. We studied the gating kinetics of Kv4.2 channels, the molecular substrate of neuronal somatodendritic A-type currents. For this purpose wild-type and mutant channels were transiently expressed in the human embryonic kidney (HEK) 293 cell line and currents were measured in the whole-cell patch-clamp configuration. 2. Kv4.2 channels inactivated from pre-open closed state(s) with a mean time constant of 959 ms at -50 mV. This closed-state inactivation was not affected by a deletion of the Kv4.2 N-terminus (Delta2-40). 3. Kv4.2 currents at +40 mV inactivated with triple-exponential kinetics. A fast component (tau = 11 ms) accounted for 73 %, an intermediate component (tau = 50 ms) for 23 % and a slow component (tau = 668 ms) for 4 % of the total decay. 4. Both the fast and the intermediate components of inactivation were slowed by a deletion of the Kv4.2 N-terminus (tau = 35 and 111 ms) and accounted for 33 and 56 %, respectively, of the total decay. The slow component was moderately accelerated by the truncation (tau = 346 ms) and accounted for 11 % of the total Kv4.2 current inactivation. 5. Recovery from open-state inactivation and recovery from closed-state inactivation occurred with similar kinetics in a strongly voltage-dependent manner. Neither recovery reaction was affected by the N-terminal truncation. 6. Kv4.2 Delta2-40 channels displayed slowed deactivation kinetics, suggesting that the N-terminal truncation leads to a stabilization of the open state. 7. Simulations with an allosteric model of inactivation, supported by the experimental data, suggested that, in response to membrane depolarization, Kv4.2 channels accumulate in the closed-inactivated state(s), from which they directly recover, bypassing the open state.
Figures
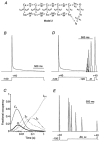
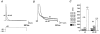
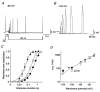
 ) and slow components (τ3, □) of inactivation at +40 mV for Kv4.2 wt (n = 13) and Δ2-40 mutant channels (n = 9). The mean percentage of the total decay accounted for by each component is indicated.
) and slow components (τ3, □) of inactivation at +40 mV for Kv4.2 wt (n = 13) and Δ2-40 mutant channels (n = 9). The mean percentage of the total decay accounted for by each component is indicated.
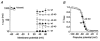
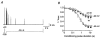
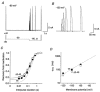

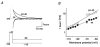
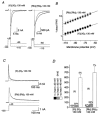
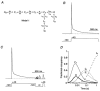
 ) and slow components (τ3, □) of inactivation at +40 mV for symmetrical K+ (n = 5) and symmetrical Rb+ (n = 6). The mean percentage of the total decay accounted for by each component is indicated.
) and slow components (τ3, □) of inactivation at +40 mV for symmetrical K+ (n = 5) and symmetrical Rb+ (n = 6). The mean percentage of the total decay accounted for by each component is indicated.References
-
- Alonso G, Widmer H. Clustering of Kv4. 2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
-
- An W F, Bowlby M R, Betty M, Cao J, Ling H P, Mendoza G, Hinson J W, Mattsson K I, Strassle B W, Trimmer J S, Rhodes K J. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Ayer R K Jr, Sigworth F J. Enhanced closed-state inactivation in a mutant Shaker K+ channel. Journal of Membrane Biology. 1997;157:215–230. - PubMed
-
- Baldwin T J, Tsaur M L, Lopez G A, Jan Y N, Jan L Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. - PubMed
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

