Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle
- PMID: 11507166
- PMCID: PMC2278759
- DOI: 10.1111/j.1469-7793.2001.00155.x
Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle
Abstract
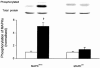
1. Exercise and contractions of isolated skeletal muscle induce phosphorylation of mitogen-activated protein kinases (MAPKs) by undefined mechanisms. The aim of the present study was to determine exercise-related triggering factors for the increased phosphorylation of MAPKs in isolated rat extensor digitorum longus (EDL) muscle. 2. Concentric or eccentric contractions, or mild or severe passive stretches were used to discriminate between effects of metabolic/ionic and mechanical alterations on phosphorylation of two MAPKs: extracellular signal-regulated kinase 1 and 2 (MAPK(erk1/2)) and stress-activated protein kinase p38 (MAPK(p38)). 3. Concentric contractions induced a 5-fold increase in MAPK(erk1/2) phosphorylation. Application of the antioxidants N-acetylcysteine (20 mM) or dithiothreitol (5 mM) suppressed concentric contraction-induced increase in MAPK(erk1/2) phosphorylation. Mild passive stretches of the muscle increased MAPK(erk1/2) phosphorylation by 1.8-fold, whereas the combination of acidosis and passive stretches resulted in a 2.8-fold increase. Neither concentric contractions, nor mild stretches nor acidosis significantly affected phosphorylation of MAPK(p38). 4. High force applied upon muscle by means of either eccentric contractions or severe passive stretches resulted in 5.7- and 9.5-fold increases of phosphorylated MAPK(erk1/2), respectively, whereas phosphorylation of MAPK(p38) increased by 7.6- and 1.9-fold (not significant), respectively. 5. We conclude that in isolated rat skeletal muscle an increase in phosphorylation of both MAPK(erk1/2) and MAPK(p38) is induced by mechanical alterations, whereas contraction-related metabolic/ionic changes (reactive oxygen species and acidosis) cause increased phosphorylation of MAPK(erk1/2) only. Thus, contraction-induced phosphorylation can be explained by the combined action of increased production of reactive oxygen species, acidification and mechanical perturbations for MAPK(erk1/2) and by high mechanical stress for MAPK(p38).
Figures

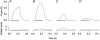

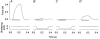
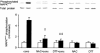
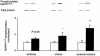
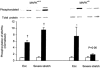
Comment in
-
How muscles know how to adapt.J Physiol. 2001 Aug 15;535(Pt 1):1. doi: 10.1111/j.1469-7793.2001.t01-2-00001.x. J Physiol. 2001. PMID: 11507152 Free PMC article. No abstract available.
Similar articles
-
Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle.Am J Physiol Cell Physiol. 2001 Feb;280(2):C352-8. doi: 10.1152/ajpcell.2001.280.2.C352. Am J Physiol Cell Physiol. 2001. PMID: 11208531
-
Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension.J Appl Physiol (1985). 2001 Aug;91(2):693-702. doi: 10.1152/jappl.2001.91.2.693. J Appl Physiol (1985). 2001. PMID: 11457783
-
Effects of insulin, contraction, and phorbol esters on mitogen-activated protein kinase signaling in skeletal muscle from lean and ob/ob mice.Diabetes. 2004 Jun;53(6):1436-44. doi: 10.2337/diabetes.53.6.1436. Diabetes. 2004. PMID: 15161746
-
Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1.J Biol Chem. 2000 Jan 14;275(2):1457-62. doi: 10.1074/jbc.275.2.1457. J Biol Chem. 2000. PMID: 10625698
-
Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport.Diabetes. 2000 Nov;49(11):1794-800. doi: 10.2337/diabetes.49.11.1794. Diabetes. 2000. PMID: 11078445
Cited by
-
Selective inhibition of ATPase activity during contraction alters the activation of p38 MAP kinase isoforms in skeletal muscle.J Cell Biochem. 2013 Jun;114(6):1445-55. doi: 10.1002/jcb.24486. J Cell Biochem. 2013. PMID: 23296747 Free PMC article.
-
Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training.J Physiol. 2014 Dec 15;592(24):5391-408. doi: 10.1113/jphysiol.2014.279950. Epub 2014 Nov 10. J Physiol. 2014. PMID: 25384788 Free PMC article.
-
Stretch-induced ERK2 phosphorylation requires PLA2 activity in skeletal myotubes.Biochem Biophys Res Commun. 2009 Aug 14;386(1):60-4. doi: 10.1016/j.bbrc.2009.05.150. Epub 2009 Jun 12. Biochem Biophys Res Commun. 2009. PMID: 19524551 Free PMC article.
-
How muscles know how to adapt.J Physiol. 2001 Aug 15;535(Pt 1):1. doi: 10.1111/j.1469-7793.2001.t01-2-00001.x. J Physiol. 2001. PMID: 11507152 Free PMC article. No abstract available.
-
Simultaneous dystrophin and dysferlin deficiencies associated with high-level expression of the coxsackie and adenovirus receptor in transgenic mice.Am J Pathol. 2006 Dec;169(6):2148-60. doi: 10.2353/ajpath.2006.060570. Am J Pathol. 2006. PMID: 17148677 Free PMC article.
References
-
- Aruoma O I, Halliwell B, Hoey B M, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology and Medicine. 1989;6:593–597. - PubMed
-
- Boppart M D, Hirshman M F, Sakamoto K, Fielding R A, Goodyear L J. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. American Journal of Physiology - Cell Physiology. 2001;280:C352–358. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
-
- Carson J A, Wei L. Integrin signaling's potential for mediating gene expression in hypertrophying skeletal muscle. Journal of Applied Physiology. 2000;88:337–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

