Human motor control consequences of thixotropic changes in muscular short-range stiffness
- PMID: 11507177
- PMCID: PMC2278763
- DOI: 10.1111/j.1469-7793.2001.00279.x
Human motor control consequences of thixotropic changes in muscular short-range stiffness
Abstract
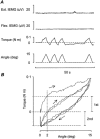
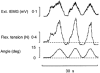
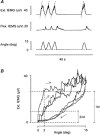
1. The primary aim of the present study was to explore whether in healthy subjects the muscle contractions required for unrestrained voluntary wrist dorsiflexions are adjusted in strength to thixotropy-dependent variations in the short-range stiffness encountered in measurements of passive torque resistance to imposed wrist dorsiflexions. 2. After a period of rest, only the first movement in a series of passive wrist dorsiflexions of moderate amplitude exhibited clear signs of short-range stiffness in the torque response. During analogous types of voluntary movements, the extensor EMG during the first movement after rest showed a steep initial rise of activity, which apparently served to compensate for the short-range stiffness. 3. The passive torque resistance to minute repetitive wrist dorsiflexions (within the range of short-range stiffness) was markedly reduced after various types of mechanical agitation. During analogous low-amplitude voluntary wrist dorsiflexions the extensor EMG signals were weaker after than before agitation. 4. Mechanical agitation also led to enhancement of passive dorsiflexion movements induced by weak constant torque pulses. In an analogous way, the movement-generating capacity of weak voluntary extensor activations (as determined by EMG recordings) was greatly enhanced by mechanical agitation. 5. The signals from a force transducer probe pressed against the wrist flexor tendons--during passive wrist dorsiflexions--revealed short-range stiffness responses which highly resembled those observed in the torque measurements, suggesting that the latter to a large extent emanated from the stretched, relaxed flexor muscles. During repetitive stereotyped voluntary wrist dorsiflexions, a close correspondence was observed between the degree of short-range stiffness as sensed by the wrist flexor tension transducer and the strength of the initial extensor activation required for movement generation. 6. The results provide evidence that the central nervous system in its control of voluntary movements takes account of and compensates for the history-dependent degree of inherent short-range stiffness of the muscles antagonistic to the prime movers.
Figures
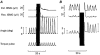
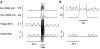

References
-
- Buchthal F, Kaiser E. The rheology of the cross-striated muscle fibre with special reference to isotonic conditions. Det Kongelige Danske Videnskabernes Selskab. Biologiske Meddelerser. 1951;21:1–307.
-
- Dietz V, Berger W. Normal and impaired regulation of muscle stiffness in gait: a new hypothesis about muscle hypertonia. Experimental Neurology. 1983;79:680–687. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

