A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection
- PMID: 11507184
- PMCID: PMC115084
- DOI: 10.1128/jvi.75.18.8390-8399.2001
A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection
Abstract
The gastrointestinal mucosa harbors the majority of the body's CD4(+) cells and appears to be uniquely susceptible to human immunodeficiency virus type 1 (HIV-1) infection. We undertook this study to examine the role of differences in chemokine receptor expression on infection of mucosal mononuclear cells (MMCs) and peripheral blood mononuclear cells (PBMCs) by R5- and X4-tropic HIV-1. We performed in vitro infections of MMCs and PBMCs with R5- and X4-tropic HIV-1, engineered to express murine CD24 on the infected cell's surface, allowing for quantification of HIV-infected cells and their phenotypic characterization. A greater percentage of MMCs than PBMCs are infected by both R5- and X4-tropic HIV-1. Significant differences exist in terms of chemokine receptor expression in the blood and gastrointestinal mucosa; mucosal cells are predominantly CCR5(+) CXCR4(+), while these cells make up less than 20% of the peripheral blood cells. It is this cell population that is most susceptible to infection with both R5- and X4-tropic HIV-1 in both compartments. Regardless of whether viral isolates were derived from the blood or mucosa of HIV-1-infected patients, HIV-1 p24 production was greater in MMCs than in PBMCs. Further, the chemokine receptor tropism of these patient-derived viral isolates did not differ between compartments. We conclude that, based on these findings, the gastrointestinal mucosa represents a favored target for HIV-1, in part due to its large population of CXCR4(+) CCR5(+) target cells and not to differences in the virus that it contains.
Figures

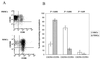
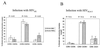


References
-
- Akbar A N, Terry L, Timms A, Beverley P C, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. - PubMed
-
- Al-Mulla W, Church D, Gill M J. Phenotypic variations and switches in HIV isolated from the blood and gastrointestinal tissues of patients with HIV-1 infection. J Med Virol. 1997;52:31–34. - PubMed
-
- Anton P A, Elliott J, Poles M A, McGowan I M, Matud J, Hultin L E, Grovit-Ferbas K, Mackay C R, Chen I S Y, Giorgi J V. Enhanced levels of functional HIV-1 coreceptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–1765. - PubMed
-
- Barnett S W, Barboza A, Wilcox C M, Forsmark C E, Levy J A. Characterization of human immunodeficiency virus type 1 strains recovered from the bowel of infected individuals. Virology. 1991;182:802–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

