An Epstein-Barr virus isolated from a lymphoblastoid cell line has a 16-kilobase-pair deletion which includes gp350 and the Epstein-Barr virus nuclear antigen 3A
- PMID: 11507201
- PMCID: PMC115101
- DOI: 10.1128/jvi.75.18.8556-8568.2001
An Epstein-Barr virus isolated from a lymphoblastoid cell line has a 16-kilobase-pair deletion which includes gp350 and the Epstein-Barr virus nuclear antigen 3A
Abstract
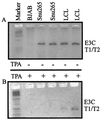
Epstein-Barr virus (EBV) is associated with human cancers, including nasopharyngeal carcinoma, Burkitt's lymphoma, gastric carcinoma and, somewhat controversially, breast carcinoma. EBV infects and efficiently transforms human primary B lymphocytes in vitro. A number of EBV-encoded genes are critical for EBV-mediated transformation of human B lymphocytes. In this study we show that an EBV-infected lymphoblastoid cell line obtained from the spontaneous outgrowth of B cells from a leukemia patient contains a deletion, which involves a region of approximately 16 kbp. This deletion encodes major EBV genes involved in both infection and transformation of human primary B lymphocytes and includes the glycoprotein gp350, the entire open reading frame of EBNA3A, and the amino-terminal region of EBNA3B. A fusion protein created by this deletion, which lies between the BMRF1 early antigen and the EBNA3B latent antigen, is truncated immediately downstream of the junction 21 amino acids into the region of the EBNA3B sequence, which is out of frame with respect to the EBNA3B protein sequence, and indicates that EBNA3B is not expressed. The fusion is from EBV coordinate 80299 within the BMRF1 sequence to coordinate 90998 in the EBNA3B sequence. Additionally, we have shown that there is no detectable induction in viral replication observed when SNU-265 is treated with phorbol esters, and no transformants were detected when supernatant is used to infect primary B lymphocytes after 8 weeks in culture. Therefore, we have identified an EBV genome with a major deletion in critical genes involved in mediating EBV infection and the transformation of human primary B lymphocytes that is incompetent for replication of this naturally occurring EBV isolate.
Figures
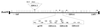







References
-
- Aman P, Lewin N, Nordstrom M, Klein G. EBV-activation of human B-lymphocytes. Curr Top Microbiol Immunol. 1986;132:266–271. - PubMed
-
- Baer R, Banker A, Biggin M, Deininger P, Farrell P, Gibson T, Hatful G, Satchwell S, Seguin C, Tuffnell P, Barrell B. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. - PubMed
-
- Bister K, Yamamoto N, zur Hausen H. Differential inducibility of Epstein-Barr virus in cloned, non-producer Raji cells. Int J Cancer. 1979;23:818–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

