Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis
- PMID: 11509666
- PMCID: PMC87342
- DOI: 10.1128/MCB.21.18.6233-6242.2001
Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis
Abstract
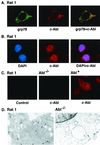
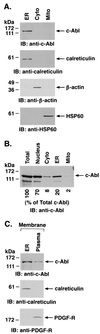
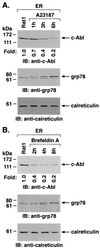
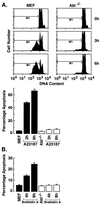
The ubiquitously expressed c-Abl tyrosine kinase localizes to the nucleus and cytoplasm. Using confocal microscopy, we demonstrated that c-Abl colocalizes with the endoplasmic reticulum (ER)-associated protein grp78. Expression of c-Abl in the ER was confirmed by immunoelectron microscopy. Subcellular fractionation studies further indicate that over 20% of cellular c-Abl is detectable in the ER. The results also demonstrate that induction of ER stress with calcium ionophore A23187, brefeldin A, or tunicamycin is associated with translocation of ER-associated c-Abl to mitochondria. In concert with targeting of c-Abl to mitochondria, cytochrome c is released in the response to ER stress by a c-Abl-dependent mechanism, and ER stress-induced apoptosis is attenuated in c-Abl-deficient cells. These findings indicate that c-Abl is involved in signaling from the ER to mitochondria and thereby the apoptotic response to ER stress.
Figures














References
-
- Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature. 1999;399:809–813. - PubMed
-
- Baskaran R, Wood L D, Whitaker L L, Xu Y, Barlow C, Canman C E, Morgan S E, Baltimore D, Wynshaw-Boris A, Kastan M B, Wang J Y J. Ataxia telangiectasia mutant protein activates c-abl tyrosine kinase in response to ionizing radiation. Nature. 1997;387:516–519. - PubMed
-
- Dricu A, Carlberg M, Wang M, Larsson O. Inhibition of N-linked glycosylation using tunicamycin causes cell death in malignant cells: role of down-regulation of the insulin-like growth factor 1 receptor in induction of apoptosis. Cancer Res. 1997;57:543–548. - PubMed
-
- Frangioni J V, Beahm P H, Shifrin V, Jost C A, Neel B G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. - PubMed
-
- Gething M, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
