DnaK chaperone-mediated control of activity of a sigma(32) homolog (RpoH) plays a major role in the heat shock response of Agrobacterium tumefaciens
- PMID: 11514513
- PMCID: PMC95412
- DOI: 10.1128/JB.183.18.5302-5310.2001
DnaK chaperone-mediated control of activity of a sigma(32) homolog (RpoH) plays a major role in the heat shock response of Agrobacterium tumefaciens
Abstract
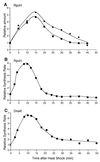
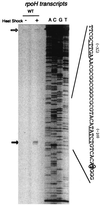
RpoH (Escherichia coli sigma(32) and its homologs) is the central regulator of the heat shock response in gram-negative proteobacteria. Here we studied salient regulatory features of RpoH in Agrobacterium tumefaciens by examining its synthesis, stability, and activity while increasing the temperature from 25 to 37 degrees C. Heat induction of RpoH synthesis occurred at the level of transcription from an RpoH-dependent promoter, coordinately with that of DnaK, and followed by an increase in the RpoH level. Essentially normal induction of heat shock proteins was observed even with a strain that was unable to increase the RpoH level upon heat shock. Moreover, heat-induced accumulation of dnaK mRNA occurred without protein synthesis, showing that preexisting RpoH was sufficient for induction of the heat shock response. These results suggested that controlling the activity, rather than the amount, of RpoH plays a major role in regulation of the heat shock response. In addition, increasing or decreasing the DnaK-DnaJ chaperones specifically reduced or enhanced the RpoH activity, respectively. On the other hand, the RpoH protein was normally stable and remained stable during the induction phase but was destabilized transiently during the adaptation phase. We propose that the DnaK-mediated control of RpoH activity plays a primary role in the induction of heat shock response in A. tumefaciens, in contrast to what has been found in E. coli.
Figures





Similar articles
-
Differential and independent roles of a sigma(32) homolog (RpoH) and an HrcA repressor in the heat shock response of Agrobacterium tumefaciens.J Bacteriol. 1999 Dec;181(24):7509-15. doi: 10.1128/JB.181.24.7509-7515.1999. J Bacteriol. 1999. PMID: 10601208 Free PMC article.
-
Regulatory conservation and divergence of sigma32 homologs from gram-negative bacteria: Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Agrobacterium tumefaciens.J Bacteriol. 1998 May;180(9):2402-8. doi: 10.1128/JB.180.9.2402-2408.1998. J Bacteriol. 1998. PMID: 9573192 Free PMC article.
-
Isolation and sequence analysis of rpoH genes encoding sigma 32 homologs from gram negative bacteria: conserved mRNA and protein segments for heat shock regulation.Nucleic Acids Res. 1995 Nov 11;23(21):4383-90. Nucleic Acids Res. 1995. PMID: 7501460 Free PMC article.
-
The heat shock response of Escherichia coli.Int J Food Microbiol. 2000 Apr 10;55(1-3):3-9. doi: 10.1016/s0168-1605(00)00206-3. Int J Food Microbiol. 2000. PMID: 10791710 Review.
-
Microbial molecular chaperones.Adv Microb Physiol. 2001;44:93-140. doi: 10.1016/s0065-2911(01)44012-4. Adv Microb Physiol. 2001. PMID: 11407116 Review.
Cited by
-
Expression of Two RpoH Sigma Factors in Sinorhizobium meliloti upon Heat Shock.Microbes Environ. 2017 Dec 27;32(4):394-397. doi: 10.1264/jsme2.ME17087. Epub 2017 Dec 2. Microbes Environ. 2017. PMID: 29199214 Free PMC article.
-
Heat shock proteome of Agrobacterium tumefaciens: evidence for new control systems.J Bacteriol. 2002 Mar;184(6):1772-8. doi: 10.1128/JB.184.6.1772-1778.2002. J Bacteriol. 2002. PMID: 11872730 Free PMC article.
-
Replicon-specific regulation of small heat shock genes in Agrobacterium tumefaciens.J Bacteriol. 2004 Oct;186(20):6824-9. doi: 10.1128/JB.186.20.6824-6829.2004. J Bacteriol. 2004. PMID: 15466035 Free PMC article.
-
The heat shock genes dnaK, dnaJ, and grpE are involved in regulation of putisolvin biosynthesis in Pseudomonas putida PCL1445.J Bacteriol. 2005 Sep;187(17):5967-76. doi: 10.1128/JB.187.17.5967-5976.2005. J Bacteriol. 2005. PMID: 16109938 Free PMC article.
-
sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti.J Bacteriol. 2005 Dec;187(23):7931-44. doi: 10.1128/JB.187.23.7931-7944.2005. J Bacteriol. 2005. PMID: 16291666 Free PMC article.
References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley; 1987.
-
- Babst M, Hennecke H, Fischer H M. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol Microbiol. 1996;19:827–839. - PubMed
-
- Blaszczak A, Georgopoulos C, Liberek K. On the mechanism of FtsH-dependent degradation of the ς32 transcriptional regulator of Escherichia coli and the role of the DnaK chaperone machine. Mol Microbiol. 1999;31:157–166. - PubMed
-
- Bukau B, Horwich A L. The HSP70 and HSP60 chaperone machines. Cell. 1998;92:351–366. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

