Directed evolution of biphenyl dioxygenase: emergence of enhanced degradation capacity for benzene, toluene, and alkylbenzenes
- PMID: 11514531
- PMCID: PMC95430
- DOI: 10.1128/JB.183.18.5441-5444.2001
Directed evolution of biphenyl dioxygenase: emergence of enhanced degradation capacity for benzene, toluene, and alkylbenzenes
Abstract
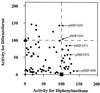
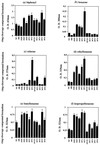
Biphenyl dioxygenase (Bph Dox) catalyzes the initial oxygenation of biphenyl and related compounds. Bph Dox is a multicomponent enzyme in which a large subunit (encoded by the bphA1 gene) is significantly responsible for substrate specificity. By using the process of DNA shuffling of bphA1 of Pseudomonas pseudoalcaligenes KF707 and Burkholderia cepacia LB400, a number of evolved Bph Dox enzymes were created. Among them, an Escherichia coli clone expressing chimeric Bph Dox exhibited extremely enhanced benzene-, toluene-, and alkylbenzene-degrading abilities. In this evolved BphA1, four amino acids (H255Q, V258I, G268A, and F277Y) were changed from the KF707 enzyme to those of the LB400 enzyme. Subsequent site-directed mutagenesis allowed us to determine the amino acids responsible for the degradation of monocyclic aromatic hydrocarbons.
Figures

References
-
- Batie C J, Ballou D P, Correll C J. Phthalate dioxygenase reductase and related flavin-iron-sulfur containing electron transferases. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 544–554.
-
- Cho M C, Kang D-O, Yoon B D, Lee K. Toluene degradation pathway from Pseudomonas putida F1: substrate specificity and gene induction by 1-substituted benzenes. J Ind Microbiol Biotechnol. 2000;25:163–170.
-
- Ensley B D, Ratzkin B J, Ossulund T D, Simon M J, Wackett L P, Gibson D T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983;22:167–169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

