Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells
- PMID: 11514600
- PMCID: PMC2193499
- DOI: 10.1084/jem.194.4.427
Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells
Abstract
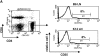
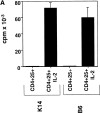
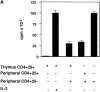
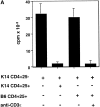
CD4(+)25(+) T cells are a unique population of immunoregulatory T cells which are critical for the prevention of autoimmunity. To address the thymic selection of these cells we have used two models of attenuated thymic deletion. In K14-A(beta)(b) mice, major histocompatibility complex (MHC) class II I-A(b) expression is limited to thymic cortical epithelium and deletion by hematopoietic antigen-presenting cells does not occur. In H2-DMalpha-deficient mice, MHC class II molecules contain a limited array of self-peptides resulting in inefficient clonal deletion. We find that CD4(+)25(+) T cells are present in the thymus and periphery of K14-A(beta)(b) and H2-DMalpha-deficient mice and, like their wild-type counterparts, suppress the proliferation of cocultured CD4(+)25(-) effector T cells. In contrast, CD4(+)25(+) T cells from MHC class II-deficient mice do not suppress responder CD4(+) T cells in vitro or in vivo. Thus, development of regulatory CD4(+)25(+) T cells is dependent on MHC class II-positive thymic cortical epithelium. Furthermore, analysis of the specificities of CD4(+)25(+) T cells in K14-A(beta)(b) and H2-DMalpha-deficient mice suggests that a subset of CD4(+)25(+) T cells is subject to negative selection on hematopoietic antigen-presenting cells.
Figures

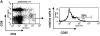
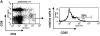
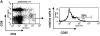
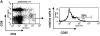



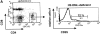
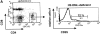
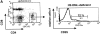
References
-
- Benoist C., Mathis D. Positive selection of the T cell repertoirewhere and when does it occur? Cell. 1989;58:1027–1033. - PubMed
-
- Berg L.J., Pullen A.M., Fazekas de St B., Groth D., Mathis C., Benoist, Davis M.M. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. - PubMed
-
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. - PubMed
-
- Lo D., Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986;319:672–675. - PubMed
-
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R.H., Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988;53:627–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

