Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense
- PMID: 11514607
- PMCID: PMC2193502
- DOI: 10.1084/jem.194.4.519
Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense
Abstract
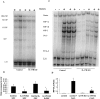
Bacterial pneumonia is an increasing complication of HIV infection and inversely correlates with the CD4(+) lymphocyte count. Interleukin (IL)-17 is a cytokine produced principally by CD4(+) T cells, which induces granulopoiesis via granulocyte colony-stimulating factor (G-CSF) production and induces CXC chemokines. We hypothesized that IL-17 receptor (IL-17R) signaling is critical for G-CSF and CXC chemokine production and lung host defenses. To test this, we used a model of Klebsiella pneumoniae lung infection in mice genetically deficient in IL-17R or in mice overexpressing a soluble IL-17R. IL-17R-deficient mice were exquisitely sensitive to intranasal K. pneumoniae with 100% mortality after 48 h compared with only 40% mortality in controls. IL-17R knockout (KO) mice displayed a significant delay in neutrophil recruitment into the alveolar space, and had greater dissemination of K. pneumoniae compared with control mice. This defect was associated with a significant reduction in steady-state levels of G-CSF and macrophage inflammatory protein (MIP)-2 mRNA and protein in the lung in response to the K. pneumoniae challenge in IL-17R KO mice. Thus, IL-17R signaling is critical for optimal production of G-CSF and MIP-2 and local control of pulmonary K. pneumoniae infection. These data support impaired IL-17R signaling as a potential mechanism by which deficiency of CD4 lymphocytes predisposes to bacterial pneumonia.
Figures



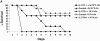
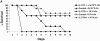
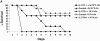





References
-
- Tumbarello M., Tacconelli E., de Gaetano K., Ardito F., Pirronti T., Cauda R., Ortona L. Bacterial pneumonia in HIV-infected patientsanalysis of risk factors and prognostic indicators. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1998;18:39–45. - PubMed
-
- Wallace J.M., Hansen N.I., Lavange L., Glassroth J., Browdy B.L., Rosen M.J., Kvale P.A., Mangura B.T., Reichman L.B., Hopewell P.C. Respiratory disease trends in the pulmonary complications of HIV infection study cohort. Pulmonary complications of HIV infection study group. Am. J. Respir. Crit. Care Med. 1997;155:72–80. - PubMed
-
- Twigg H.L., III, Spain B.A., Soliman D.M., Bowen L.K., Heidler K.M., Wilkes D.S. Impaired IgG production in the lungs of HIV-infected individuals. Cell. Immunol. 1996;170:127–133. - PubMed
-
- Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.-M., Painter S.L., Comeau M.R., Cohen J.I., Spriggs M.K. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to novel cytokine receptor. Immunity. 1995;3:811–821. - PubMed
-
- Yao Z., Painter S.L., Fanslow W.C., Ulrich D., Macduff B.M., Spriggs M.K., Armitage R.J. Human IL-17a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–5486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

