Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes
- PMID: 11514628
- PMCID: PMC58606
- DOI: 10.1091/mbc.12.8.2453
Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes
Abstract
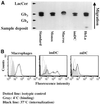
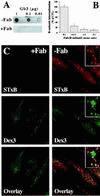
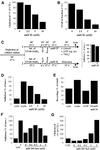
In HeLa cells, Shiga toxin B-subunit is transported from the plasma membrane to the endoplasmic reticulum, via early endosomes and the Golgi apparatus, circumventing the late endocytic pathway. We describe here that in cells derived from human monocytes, i.e., macrophages and dendritic cells, the B-subunit was internalized in a receptor-dependent manner, but retrograde transport to the biosynthetic/secretory pathway did not occur and part of the internalized protein was degraded in lysosomes. These differences correlated with the observation that the B-subunit associated with Triton X-100-resistant membranes in HeLa cells, but not in monocyte-derived cells, suggesting that retrograde targeting to the biosynthetic/secretory pathway required association with specialized microdomains of biological membranes. In agreement with this hypothesis we found that in HeLa cells, the B-subunit resisted extraction by Triton X-100 until its arrival in the target compartments of the retrograde pathway, i.e., the Golgi apparatus and the endoplasmic reticulum. Furthermore, destabilization of Triton X-100-resistant membranes by cholesterol extraction potently inhibited B-subunit transport from early endosomes to the trans-Golgi network, whereas under the same conditions, recycling of transferrin was not affected. Our data thus provide first evidence for a role of lipid asymmetry in membrane sorting at the interface between early endosomes and the trans-Golgi network.
Figures







References
-
- Abe A, Inokuchi J, Jimbo M, Shimeno H, Nagamatsu A, Shayman JA, Shukla GS, Radin NS. Improved inhibitors of glucosylceramide synthase. J Biochem. 1992;111:191–196. - PubMed
-
- Arab S, Lingwood CA. Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramide fatty acid isoform traffic. J Cell Physiol. 1998;177:646–660. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

