Substitution of conserved methionines by leucines in chloroplast small heat shock protein results in loss of redox-response but retained chaperone-like activity
- PMID: 11514669
- PMCID: PMC2253196
- DOI: 10.1110/ps.11301
Substitution of conserved methionines by leucines in chloroplast small heat shock protein results in loss of redox-response but retained chaperone-like activity
Abstract
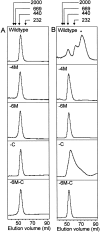
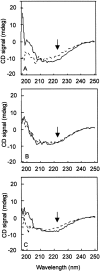
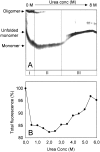
During evolution of land plants, a specific motif occurred in the N-terminal domain of the chloroplast-localized small heat shock protein, Hsp21: a sequence with highly conserved methionines, which is predicted to form an amphipathic alpha-helix with the methionines situated along one side. The functional role of these conserved methionines is not understood. We have found previously that treatment, which causes methionine sulfoxidation in Hsp21, also leads to structural changes and loss of chaperone-like activity. Here, mutants of Arabidopsis thaliana Hsp21 protein were created by site-directed mutagenesis, whereby conserved methionines were substituted by oxidation-resistant leucines. Mutants lacking the only cysteine in Hsp21 were also created. Protein analyses by nondenaturing electrophoresis, size exclusion chromatography, and circular dichroism proved that sulfoxidation of the four highly conserved methionines (M49, M52, M55, and M59) is responsible for the oxidation-induced conformational changes in the Hsp21 oligomer. In contrast, the chaperone-like activity was not ultimately dependent on the methionines, because it was retained after methionine-to-leucine substitution. The functional role of the conserved methionines in Hsp21 may be to offer a possibility for redox control of chaperone-like activity and oligomeric structure dynamics.
Figures




Similar articles
-
The chaperone-like activity of a small heat shock protein is lost after sulfoxidation of conserved methionines in a surface-exposed amphipathic alpha-helix.Biochim Biophys Acta. 2001 Feb 9;1545(1-2):227-37. doi: 10.1016/s0167-4838(00)00280-6. Biochim Biophys Acta. 2001. PMID: 11342048
-
Conserved methionines in chloroplasts.Biochim Biophys Acta. 2005 Jan 17;1703(2):191-202. doi: 10.1016/j.bbapap.2004.09.001. Biochim Biophys Acta. 2005. PMID: 15680227 Review.
-
Methionine sulfoxidation of the chloroplast small heat shock protein and conformational changes in the oligomer.Protein Sci. 1999 Nov;8(11):2506-12. doi: 10.1110/ps.8.11.2506. Protein Sci. 1999. PMID: 10595556 Free PMC article.
-
A peptide methionine sulfoxide reductase highly expressed in photosynthetic tissue in Arabidopsis thaliana can protect the chaperone-like activity of a chloroplast-localized small heat shock protein.Plant J. 2002 Mar;29(5):545-53. doi: 10.1046/j.1365-313x.2002.029005545.x. Plant J. 2002. PMID: 11874568
-
The roles of conditional disorder in redox proteins.Curr Opin Struct Biol. 2013 Jun;23(3):436-42. doi: 10.1016/j.sbi.2013.02.006. Epub 2013 Mar 13. Curr Opin Struct Biol. 2013. PMID: 23477949 Free PMC article. Review.
Cited by
-
Protein Methionine Sulfoxide Dynamics in Arabidopsis thaliana under Oxidative Stress.Mol Cell Proteomics. 2015 May;14(5):1217-29. doi: 10.1074/mcp.M114.043729. Epub 2015 Feb 18. Mol Cell Proteomics. 2015. PMID: 25693801 Free PMC article.
-
Chemical cross-linking of the chloroplast localized small heat-shock protein, Hsp21, and the model substrate citrate synthase.Protein Sci. 2007 Jul;16(7):1464-78. doi: 10.1110/ps.072831607. Epub 2007 Jun 13. Protein Sci. 2007. PMID: 17567739 Free PMC article.
-
Proteome-derived peptide libraries allow detailed analysis of the substrate specificities of N(alpha)-acetyltransferases and point to hNaa10p as the post-translational actin N(alpha)-acetyltransferase.Mol Cell Proteomics. 2011 May;10(5):M110.004580. doi: 10.1074/mcp.M110.004580. Epub 2011 Mar 7. Mol Cell Proteomics. 2011. PMID: 21383206 Free PMC article.
-
Methionine mutations of outer membrane protein X influence structural stability and beta-barrel unfolding.PLoS One. 2013 Nov 12;8(11):e79351. doi: 10.1371/journal.pone.0079351. eCollection 2013. PLoS One. 2013. PMID: 24265768 Free PMC article.
-
Sulfur assimilation and the role of sulfur in plant metabolism: a survey.Photosynth Res. 2004;79(3):331-48. doi: 10.1023/B:PRES.0000017196.95499.11. Photosynth Res. 2004. PMID: 16328799
References
-
- Berlett, B.S. and Stadtman, E.R. 1997. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272 20313–20316. - PubMed
-
- Bova, M.P., Yaron, O., Huang, Q., Ding, L., Haley, D.A., Stewart, P.L., and Horwitz, J. 1999. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl. Acad. Sci. 96 6137–6142. - PMC - PubMed
-
- Buchner, J., Grallert, H., and Jakob, U. 1998. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 290 323–338. - PubMed
-
- Caspers, G.J., Leunissen, J.A.M., and De Jong, W.W. 1995. The expanding small heat-shock protein family, and structure predictions of the conserved "alpha-crystallin domain". J. Mol. Evol. 40 238–248. - PubMed
-
- Chen, Q. and Vierling, E. 1991. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol. Gen. Genet. 226 425–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

