Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons
- PMID: 11517244
- PMCID: PMC6763106
- DOI: 10.1523/JNEUROSCI.21-17-06553.2001
Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons
Abstract
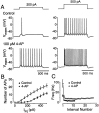
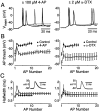
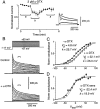
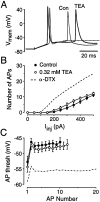
Many neurons transduce synaptic inputs into action potentials (APs) according to rules that reflect their intrinsic membrane properties. Voltage-gated potassium channels, being numerous and diverse constituents of neuronal membrane, are important participants in neuronal excitability and thus in synaptic integration. Here we address the role of dendrotoxin-sensitive "D-type" potassium channels in the excitability of large pyramidal neurons in layer 5 of the rat neocortex. Low concentrations of 4-aminopyridine or alpha-dendrotoxin (alpha-DTX) dramatically increased excitability: the firing threshold for action potentials was hyperpolarized by 4-8 mV, and the firing frequency during a 1-sec-long 500 pA somatic current step was doubled. In nucleated outside-out patches pulled from the soma, alpha-DTX reversibly blocked a slowly inactivating potassium current that comprised approximately 6% of the total. This current first turned on at voltages just hyperpolarized to the threshold for spiking and activated steeply with depolarization. By assaying alpha-DTX-sensitive current in outside-out patches pulled from the axon and primary apical dendrite, it was found that this current was concentrated near the soma. We conclude that alpha-DTX-sensitive channels are present on large layer 5 pyramidal neurons at relatively low density, but their strategic location close to the site of action potential initiation in the axon may ensure that they have a disproportionate effect on neuronal excitability. Modulation of this class of channel would generate a powerful upregulation or downregulation of neuronal output after the integration of synaptic inputs.
Figures



References
-
- Albert JL, Nerbonne JM. Calcium-independent depolarization-activated potassium currents in superior colliculus-projecting rat visual cortical neurons. J Neurophysiol. 1995;73:2163–2178. - PubMed
-
- Bekkers JM. Distribution of slow AHP channels on hippocampal CA1 pyramidal neurons. J Neurophysiol. 2000c;83:1756–1759. - PubMed
-
- Berger T, Larkum ME, Lüscher H-R. High Ih channel density in the distal apical dendrite of Layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
