Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons
- PMID: 11517259
- PMCID: PMC6763116
- DOI: 10.1523/JNEUROSCI.21-17-06694.2001
Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons
Abstract
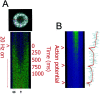
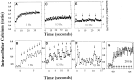
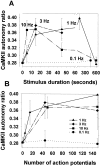
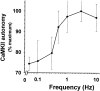
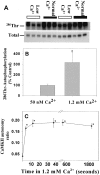
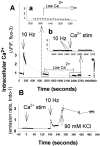
Autonomous activation of calcium-calmodulin kinase (CaMKII) has been proposed as a molecular mechanism for decoding Ca(2+) spike frequencies resulting from action potential firing, but this has not been investigated in intact neurons. This was studied in mouse DRG neurons in culture using confocal measurements of [Ca(2+)](i) and biochemical measurements of CaMKII autophosphorylation and autonomous activity. Using electrical stimulation at different frequencies, we find that CaMKII autonomous activity reached near maximal levels after approximately 45 impulses, regardless of firing frequency (1-10 Hz), and autonomous activity declined with prolonged stimulation. Frequency-dependent activation of CaMKII was limited to spike frequencies in the range of 0.1-1 Hz, despite marked increases in [Ca(2+)](i) at higher frequencies (1-30 Hz). The high levels of autonomous activity measured before stimulation and the relatively long duration of Ca(2+) spikes induced by action potentials ( approximately 300 msec) are consistent with the lower frequency range of action potential decoding by CaMKII. The high autonomous activity under basal conditions was associated with extracellular [Ca(2+)], independently from changes in [Ca(2+)](i), and unrelated to synaptic or spontaneous impulse activity. CaMKII autonomous activity in response to brief bursts of action potentials correlated better with the frequency of Ca(2+) transients than with the concentration of [Ca(2+)](i). In conclusion, CaMKII may decode frequency-modulated responses between 0.1 and 1 Hz in these neurons, but other mechanisms may be required to decode higher frequencies. Alternatively, CaMKII may mediate high-frequency responses in subcellular microdomains in which the enzyme is maintained at a low level of autonomous activity or the Ca(2+) transients have faster kinetics.
Figures



Similar articles
-
CaMKII inactivation by extracellular Ca(2+) depletion in dorsal root ganglion neurons.Cell Calcium. 2006 May;39(5):445-54. doi: 10.1016/j.ceca.2006.01.005. Epub 2006 Mar 7. Cell Calcium. 2006. PMID: 16519936 Free PMC article.
-
Autophosphorylated CaMKII Facilitates Spike Propagation in Rat Optic Nerve.J Neurosci. 2018 Sep 12;38(37):8087-8105. doi: 10.1523/JNEUROSCI.0078-18.2018. Epub 2018 Aug 3. J Neurosci. 2018. PMID: 30076212 Free PMC article.
-
P2Y1 and P2X7 receptors induce calcium/calmodulin-dependent protein kinase II phosphorylation in cerebellar granule neurons.Eur J Neurosci. 2006 Jun;23(11):2999-3013. doi: 10.1111/j.1460-9568.2006.04832.x. Eur J Neurosci. 2006. PMID: 16819989
-
Regulation and role of brain calcium/calmodulin-dependent protein kinase II.Neurochem Int. 1992 Dec;21(4):469-97. doi: 10.1016/0197-0186(92)90080-b. Neurochem Int. 1992. PMID: 1338943 Review.
-
Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II.Biochem J. 2002 Jun 15;364(Pt 3):593-611. doi: 10.1042/BJ20020228. Biochem J. 2002. PMID: 11931644 Free PMC article. Review.
Cited by
-
The neuronal stimulation-transcription coupling map.Curr Opin Neurobiol. 2019 Dec;59:87-94. doi: 10.1016/j.conb.2019.05.001. Epub 2019 Jun 1. Curr Opin Neurobiol. 2019. PMID: 31163285 Free PMC article. Review.
-
CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels.Nat Neurosci. 2006 Jan;9(1):41-9. doi: 10.1038/nn1602. Epub 2005 Dec 11. Nat Neurosci. 2006. PMID: 16341213 Free PMC article.
-
Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells.J Physiol. 2006 Oct 1;576(Pt 1):163-78. doi: 10.1113/jphysiol.2006.114876. Epub 2006 Jul 20. J Physiol. 2006. PMID: 16857709 Free PMC article.
-
Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle.J Physiol. 2003 Nov 15;553(Pt 1):303-9. doi: 10.1113/jphysiol.2003.054171. Epub 2003 Oct 17. J Physiol. 2003. PMID: 14565989 Free PMC article. Clinical Trial.
-
Mechanisms of dendritic maturation.Mol Neurobiol. 2004 Jun;29(3):303-20. doi: 10.1385/MN:29:3:303. Mol Neurobiol. 2004. PMID: 15181241 Review.
References
-
- Bayer K-U, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous γ- and δ-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Mol Brain Res. 1999;70:147–154. - PubMed
-
- Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM Kinase II. J Biol Chem. 1999;274:22713–22722. - PubMed
-
- Coomber CJ. Site-selective autophosphorylation of Ca2+/calmodulin-dependence protein kinase II as a synaptic encoding mechanism. Neural Comput. 1998;10:1653–1678. - PubMed
-
- De Koninck P, Schulman H. Sensitivity of CaMKII to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
