The cortical representation of the hand in macaque and human area S-I: high resolution optical imaging
- PMID: 11517270
- PMCID: PMC6763104
- DOI: 10.1523/JNEUROSCI.21-17-06820.2001
The cortical representation of the hand in macaque and human area S-I: high resolution optical imaging
Abstract
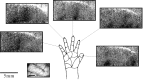
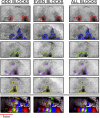
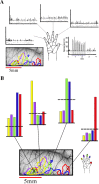
High-resolution images of the somatotopic hand representation in macaque monkey primary somatosensory cortex (area S-I) were obtained by optical imaging based on intrinsic signals. To visualize somatotopic maps, we imaged optical responses to mild tactile stimulation of each individual fingertip. The activation evoked by stimulation of a single finger was strongest in a narrow transverse band ( approximately 1 x 4 mm) across the postcentral gyrus. As expected, a sequential organization of these bands was found. However, a significant overlap, especially for the activated areas of fingers 3-5, was found. Surprisingly, in addition to the finger-specific domains, we found that for each of the fingers, weak stimulation activated also a second "common patch" of cortex, located just medially to the representation of the finger. These results were confirmed by targeted multiunit and single-unit recordings guided by the optical maps. The maps remained very stable over many hours of recording. By optimizing the imaging procedures, we were able to obtain the functional maps extremely rapidly (e.g., the map of five fingers in the macaque monkey could be obtained in as little as 5 min). Furthermore, we describe the intraoperative optical imaging of the hand representation in the human brain during neurosurgery and then discuss the implications of the present results for the spatial resolution accomplishable by other neuroimaging techniques, relying on responses of the microcirculation to sensory-evoked electrical activity. This study demonstrates the feasibility of using high-resolution optical imaging to explore reliably short- and long-term plasticity of cortical representations, as well as for applications in the clinical setting.
Figures












References
-
- Bakin JS, Kwon MC, Masino SA, Weinberger NM, Frostig RD. Suprathreshold auditory cortex activation visualized by intrinsic signal optical imaging. Cereb Cortex. 1996;6:120–130. - PubMed
-
- Bonhoeffer T, Kim DS, Malonek D, Shoham D, Grinvald A. Optical imaging of the layout of functional domains in area 17 and across the area 17/18 border in cat visual cortex. Eur J Neurosci. 1995;7:1973–1988. - PubMed
-
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. - PubMed
-
- Cannestra AF, Black KL, Martin NA, Cloughesy T, Burton JS, Rubinstein E, Woods RP, Toga AW. Topographical and temporal specificity of human intraoperative optical intrinsic signals. NeuroReport. 1998;9:2557–2563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
