Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease
- PMID: 11517273
- PMCID: PMC6763089
- DOI: 10.1523/JNEUROSCI.21-17-06853.2001
Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease
Abstract
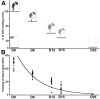
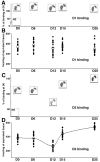
The concept of a threshold of dopamine (DA) depletion for onset of Parkinson's disease symptoms, although widely accepted, has, to date, not been determined experimentally in nonhuman primates in which a more rigorous definition of the mechanisms responsible for the threshold effect might be obtained. The present study was thus designed to determine (1) the relationship between Parkinsonian symptom appearance and level of degeneration of the nigrostriatal pathway and (2) the concomitant presynaptic and postsynaptic striatal response to the denervation, in monkeys treated chronically with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine according to a regimen that produces a progressive Parkinsonian state. The kinetics of the nigrostriatal degeneration described allow the determination of the critical thresholds associated to symptom appearance, these were a loss of 43.2% of tyrosine hydroxylase-immunopositive neurons at the nigral level and losses of 80.3 and 81.6% DA transporter binding and DA content, respectively, at the striatal level. Our data argue against the concept that an increase in DA metabolism could act as an efficient adaptive mechanism early in the disease progress. Surprisingly, the D(2)-like DA receptor binding showed a biphasic regulation in relation to the level of striatal dopaminergic denervation, i.e., an initial decrease in the presymptomatic period was followed by an upregulation of postsynaptic receptors commencing when striatal dopaminergic homeostasis is broken. Further in vivo follow-up of the kinetics of striatal denervation in this, and similar, experimental models is now needed with a view to developing early diagnosis tools and symptomatic therapies that might enhance endogenous compensatory mechanisms.
Figures


References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. - PubMed
-
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human Parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. - PubMed
-
- Bezard E, Boraud T, Bioulac B, Gross C. Compensatory effects of glutamatergic inputs to the substantia nigra pars compacta in experimental Parkinsonism. Neuroscience. 1997a;81:399–404. - PubMed
-
- Bezard E, Imbert C, Deloire X, Bioulac B, Gross C. A chronic MPTP model reproducing the slow evolution of Parkinson's disease: evolution of motor symptoms in the monkey. Brain Res. 1997b;766:107–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
