Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release
- PMID: 11517274
- PMCID: PMC6763105
- DOI: 10.1523/JNEUROSCI.21-17-06862.2001
Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release
Erratum in
- J Neurosci 2001 Oct 1;21(19):1a. Hall SF [corrected to Hall FS]
Abstract
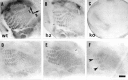
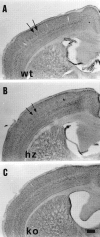
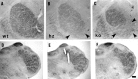
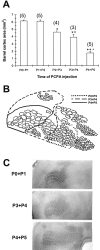
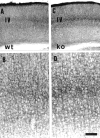
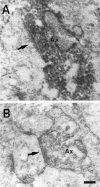
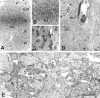
Thalamocortical neurons innervating the barrel cortex in neonatal rodents transiently store serotonin (5-HT) in synaptic vesicles by expressing the plasma membrane serotonin transporter (5-HTT) and the vesicular monoamine transporter (VMAT2). 5-HTT knock-out (ko) mice reveal a nearly complete absence of 5-HT in the cerebral cortex by immunohistochemistry, and of barrels, both at P7 and adulthood. Quantitative electron microscopy reveals that 5-HTT ko affects neither the density of synapses nor the length of synaptic contacts in layer IV. VMAT2 ko mice, completely lacking activity-dependent vesicular release of monoamines including 5-HT, also show a complete lack of 5-HT in the cortex but display largely normal barrel fields, despite sometimes markedly reduced postnatal growth. Transient 5-HTT expression is thus required for barrel pattern formation, whereas activity-dependent vesicular 5-HT release is not.
Figures



Similar articles
-
Transient uptake and storage of serotonin in developing thalamic neurons.Neuron. 1996 Nov;17(5):823-35. doi: 10.1016/s0896-6273(00)80215-9. Neuron. 1996. PMID: 8938116
-
Effects of genetic depletion of monoamines on somatosensory cortical development.Neuroscience. 2002;115(3):753-64. doi: 10.1016/s0306-4522(02)00484-0. Neuroscience. 2002. PMID: 12435414
-
Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development.Brain Res Dev Brain Res. 2004 Jun 21;150(2):151-61. doi: 10.1016/j.devbrainres.2003.02.001. Brain Res Dev Brain Res. 2004. PMID: 15158078
-
Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter.Anat Rec. 2002 Jun 1;267(2):87-93. doi: 10.1002/ar.10089. Anat Rec. 2002. PMID: 11997877 Review.
-
Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system.Front Neuroendocrinol. 1998 Jul;19(3):187-231. doi: 10.1006/frne.1998.0168. Front Neuroendocrinol. 1998. PMID: 9665836 Review.
Cited by
-
Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes.Front Cell Neurosci. 2013 Jun 19;7:93. doi: 10.3389/fncel.2013.00093. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23801939 Free PMC article.
-
Identification of genetic modifiers of behavioral phenotypes in serotonin transporter knockout rats.BMC Genet. 2010 May 7;11:37. doi: 10.1186/1471-2156-11-37. BMC Genet. 2010. PMID: 20459657 Free PMC article.
-
Developmental alterations in anxiety and cognitive behavior in serotonin transporter mutant mice.Psychopharmacology (Berl). 2014 Oct;231(21):4119-33. doi: 10.1007/s00213-014-3554-x. Epub 2014 Apr 13. Psychopharmacology (Berl). 2014. PMID: 24728652
-
An autism-associated serotonin transporter variant disrupts multisensory processing.Transl Psychiatry. 2017 Mar 21;7(3):e1067. doi: 10.1038/tp.2017.17. Transl Psychiatry. 2017. PMID: 28323282 Free PMC article.
-
GAP-43 is critical for normal development of the serotonergic innervation in forebrain.J Neurosci. 2002 May 1;22(9):3543-52. doi: 10.1523/JNEUROSCI.22-09-03543.2002. J Neurosci. 2002. PMID: 11978831 Free PMC article.
References
-
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. - PubMed
-
- Bailey CH, Chen MC, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–649. - PubMed
-
- Bell L, Madri JA. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res. 1989;65:1057–1065. - PubMed
-
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Moessner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. - PubMed
-
- Bennett-Clarke CA, Chiaia NL, Crissman RS, Rhoades RW. The source of the transient serotoninergic input to the developing visual and somatosensory cortices in rat. Neuroscience. 1991;43:163–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
