Nociceptor sensitization by extracellular signal-regulated kinases
- PMID: 11517280
- PMCID: PMC6763065
- DOI: 10.1523/JNEUROSCI.21-17-06933.2001
Nociceptor sensitization by extracellular signal-regulated kinases
Abstract
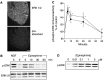
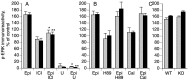
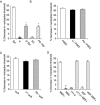
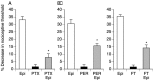
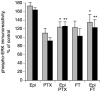
Inflammatory pain, characterized by a decrease in mechanical nociceptive threshold (hyperalgesia), arises through actions of inflammatory mediators, many of which sensitize primary afferent nociceptors via G-protein-coupled receptors. Two signaling pathways, one involving protein kinase A (PKA) and one involving the epsilon isozyme of protein kinase C (PKCepsilon), have been implicated in primary afferent nociceptor sensitization. Here we describe a third, independent pathway that involves activation of extracellular signal-regulated kinases (ERKs) 1 and 2. Epinephrine, which induces hyperalgesia by direct action at beta(2)-adrenergic receptors on primary afferent nociceptors, stimulated phosphorylation of ERK1/2 in cultured rat dorsal root ganglion cells. This was inhibited by a beta(2)-adrenergic receptor blocker and by an inhibitor of mitogen and extracellular signal-regulated kinase kinase (MEK), which phosphorylates and activates ERK1/2. Inhibitors of G(i/o)-proteins, Ras farnesyltransferases, and MEK decreased epinephrine-induced hyper-algesia. In a similar manner, phosphorylation of ERK1/2 was also decreased by these inhibitors. Local injection of dominant active MEK produced hyperalgesia that was unaffected by PKA or PKCepsilon inhibitors. Conversely, hyperalgesia produced by agents that activate PKA or PKCepsilon was unaffected by MEK inhibitors. We conclude that a Ras-MEK-ERK1/2 cascade acts independent of PKA or PKCepsilon as a novel signaling pathway for the production of inflammatory pain. This pathway may present a target for a new class of analgesic agents.
Figures
References
-
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
-
- Bailey CH, Kaang B-K, Chen M, Martin KC, Lim C-S, Casadio A, Kandel ER. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron. 1997;18:913–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
