The nuclear factor kappa B (NF-kappa B): a potential therapeutic target for estrogen receptor negative breast cancers
- PMID: 11517301
- PMCID: PMC56970
- DOI: 10.1073/pnas.151257998
The nuclear factor kappa B (NF-kappa B): a potential therapeutic target for estrogen receptor negative breast cancers
Abstract
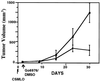
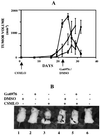
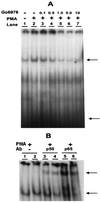
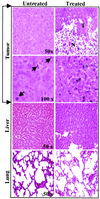
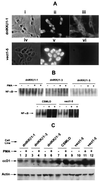
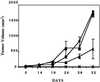
The effect of a kinase inhibitor Go6796 on growth of epidermal growth factor (EGF)-stimulated estrogen receptor negative (ER-) breast cancer cells in vivo and role of nuclear factor kappa B (NF-kappaB) on tumorogenesis have been investigated. This was studied in an animal model by implanting ER- mouse mammary epithelial tumor cells (CSMLO) in syngeneic A-J mice. (i) Local administration of Go6976 an inhibitor of protein kinases C alpha and beta inhibited growth of tumors and caused extensive necrotic degeneration and regression of the tumors without causing any microscopically detectable damage to the vital organs liver and lung. (ii) Stable expression of dominant-negative mutants of the beta subunit (dnIkkbeta) of the inhibitory kappa B (IkappaB) kinase (dnIkk) that selectively blocked activation of NF-kappaB caused loss of tumorigenic potential of CSMLO cells. Stable expression of dnIkkbeta also blocked phorbol 12-myristate 13-acetate (PMA)-induced activation of NF-kappaB and overexpression of cyclin D1, concomitantly with the loss or reduced tumorigenic potential of these cells. Thus, results from in vivo and in vitro experiments strongly suggest the involvement of NF-kappaB in ER- mammary epithelial cell-mediated tumorigenesis. We propose that blocking NF-kappaB activation not only inhibits cell proliferation, but also antagonizes the antiapoptotic role of this transcription factor in ER- breast cancer cells. Thus, NF-kappaB is a potential target for therapy of EGFR family receptor-overexpressing ER- breast cancers.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

