An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci
- PMID: 11517340
- PMCID: PMC58569
- DOI: 10.1073/pnas.191260798
An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci
Abstract
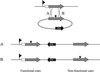
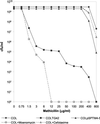
The blanket resistance of methicillin-resistant Staphylococcus aureus to all beta-lactam antibiotics--which had such a devastating impact on chemotherapy of staphylococcal infections--is related to the properties of the key component of this resistance mechanism: the "acquired" penicillin-binding protein (PBP)-2A, which has unusual low affinity for all beta-lactam antibiotics. Until now, the accepted model of resistance implied that in the presence of beta-lactam antibiotics in the surrounding medium, PBP2A must take over the biosynthesis of staphylococcal cell wall from the four native staphylococcal PBPs because the latter become rapidly acylated and inactivated at even low concentrations of the antibiotic. However, recent observations indicate that this model requires revision. Inactivation of the transglycosylase domain, but not the transpeptidase domain, of PBP2 of S. aureus prevents expression of beta-lactam resistance, despite the presence of the low-affinity PBP2A. The observations suggest that cell-wall synthesis in the presence of beta-lactam antibiotics requires the cooperative functioning of the transglycosylase domain of the native staphylococcal PBP2 and the transpeptidase domain of the PBP2A, a protein imported by S. aureus from an extra species source.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

