Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis
- PMID: 11517342
- PMCID: PMC58565
- DOI: 10.1073/pnas.191284198
Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis
Abstract
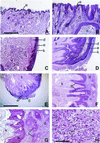
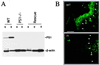
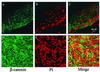
Presenilin 1 (PS1) is required for the proteolytic processing of Notch and the beta-amyloid precursor protein (APP), molecules that play pivotal roles in cell-fate determination during development and Alzheimer's disease pathogenesis, respectively. In addition, PS1 interacts with beta-catenin and promotes its turnover through independent mechanisms. Consistent with this activity, we report here that PS1 is important in controlling epidermal cell proliferation in vivo. PS1 knockout mice that are rescued through neuronal expression of human PS1 transgene develop spontaneous skin cancers. PS1-null keratinocytes exhibit higher cytosolic beta-catenin and beta-catenin/lymphoid enhancer factor-1/T cell factor (beta-catenin/LEF)-mediated signaling. This effect can be reversed by reintroducing wild-type PS1, but not a PS1 mutant active in Notch processing but defective in beta-catenin binding. Nuclear beta-catenin protein can be detected in tumors. Elevated beta-catenin/LEF signaling is correlated with activation of its downstream target cyclin D1 and accelerated entry from G(1) into S phase of the cell cycle. This report demonstrates a function of PS1 in adult tissues, and our analysis suggests that deregulation of beta-catenin pathway contributes to the skin tumor phenotype.
Figures

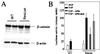
Comment in
-
From Alzheimer's disease to skin tumors: the catenin connection.Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10522-3. doi: 10.1073/pnas.211430698. Proc Natl Acad Sci U S A. 2001. PMID: 11553799 Free PMC article. No abstract available.
References
-
- Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. - PubMed
-
- Selkoe D J. Trends Cell Biol. 1998;8:447–453. - PubMed
-
- De Strooper B, Beullens M, Contreras B, Levesque L, Craessaerts K, Cordell B, Moechars D, Bollen M, Fraser P, George-Hyslop P S, Van Leuven F. J Biol Chem. 1997;272:3590–3598. - PubMed
-
- Georgakopoulos A, Marambaud P, Efthimiopoulos S, Shioi J, Cui W, Li H C, Schutte M, Gordon R, Holstein G R, Martinelli G, et al. Mol Cell. 1999;4:893–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

