A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis
- PMID: 11518726
- PMCID: PMC209397
- DOI: 10.1172/JCI12153
A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis
Abstract
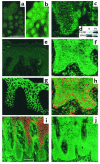
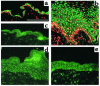
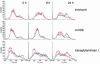
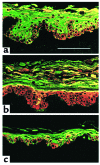
In normal epidermis, beta1 integrin expression is confined to the basal layer, whereas in hyperproliferative epidermis, integrins are also expressed in the suprabasal layers. Transgenic mice in which integrins are expressed suprabasally via the involucrin promoter have a sporadic psoriatic phenotype; however, the mechanism by which integrins contribute to the pathogenesis of psoriasis is unknown. We observed activation of mitogen-activated protein kinase (MAPK) in basal and suprabasal keratinocytes of human and transgenic mouse psoriatic lesions and healing mouse skin wounds, correlating in each case with suprabasal integrin expression. Phenotypically normal human and transgenic mouse epidermis did not contain activated MAPK. Transgene-positive keratinocytes produced more IL-1alpha than controls did, and keratinocyte MAPK could be activated by ligation of suprabasal integrins or treatment with IL-1alpha. Constitutive activation of MAPK increased the growth rate of human keratinocytes and delayed the onset of terminal differentiation, recreating many of the histological features of psoriatic epidermis. We propose that activation of MAPK by integrins, either directly or through increased IL-1alpha production, is responsible for epidermal hyperproliferation in psoriasis and wound healing, and that the sporadic phenotype of the transgenic mice may reflect the complex mechanisms by which IL-1 release and responsiveness are controlled in skin.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

