Biological degradation of 2,4,6-trinitrotoluene
- PMID: 11527999
- PMCID: PMC99030
- DOI: 10.1128/MMBR.65.3.335-352.2001
Biological degradation of 2,4,6-trinitrotoluene
Abstract
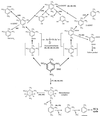
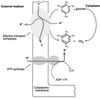
Nitroaromatic compounds are xenobiotics that have found multiple applications in the synthesis of foams, pharmaceuticals, pesticides, and explosives. These compounds are toxic and recalcitrant and are degraded relatively slowly in the environment by microorganisms. 2,4,6-Trinitrotoluene (TNT) is the most widely used nitroaromatic compound. Certain strains of Pseudomonas and fungi can use TNT as a nitrogen source through the removal of nitrogen as nitrite from TNT under aerobic conditions and the further reduction of the released nitrite to ammonium, which is incorporated into carbon skeletons. Phanerochaete chrysosporium and other fungi mineralize TNT under ligninolytic conditions by converting it into reduced TNT intermediates, which are excreted to the external milieu, where they are substrates for ligninolytic enzymes. Most if not all aerobic microorganisms reduce TNT to the corresponding amino derivatives via the formation of nitroso and hydroxylamine intermediates. Condensation of the latter compounds yields highly recalcitrant azoxytetranitrotoluenes. Anaerobic microorganisms can also degrade TNT through different pathways. One pathway, found in Desulfovibrio and Clostridium, involves reduction of TNT to triaminotoluene; subsequent steps are still not known. Some Clostridium species may reduce TNT to hydroxylaminodinitrotoluenes, which are then further metabolized. Another pathway has been described in Pseudomonas sp. strain JLR11 and involves nitrite release and further reduction to ammonium, with almost 85% of the N-TNT incorporated as organic N in the cells. It was recently reported that in this strain TNT can serve as a final electron acceptor in respiratory chains and that the reduction of TNT is coupled to ATP synthesis. In this review we also discuss a number of biotechnological applications of bacteria and fungi, including slurry reactors, composting, and land farming, to remove TNT from polluted soils. These treatments have been designed to achieve mineralization or reduction of TNT and immobilization of its amino derivatives on humic material. These approaches are highly efficient in removing TNT, and increasing amounts of research into the potential usefulness of phytoremediation, rhizophytoremediation, and transgenic plants with bacterial genes for TNT removal are being done.
Figures




References
-
- Achtnich C, Fernandes E, Bollag J-M, Knackmuss H-J, Lenke H. Covalent binding of reduced metabolites of 15N-TNT to soil organic matter during a bioremediation process analyzed by 15N NMR spectroscopy. Environ Sci Technol. 1999;33:4448–4456.
-
- Achtnich C, Lenke H. Stability of immobilized 2,4,6-trinitrotoluene metabolites in soil under long-term leaching conditions. Environ Toxicol Chem. 2001;20:280–283. - PubMed
-
- Achtnich C, Lenke H, Klaus U, Spiteller M, Knackmuss H-J. Stability of immobilized TNT derivatives in soil as a function of nitro group reduction. Environ Sci Technol. 2000;34:3698–3704.
-
- Achtnich C, Pfortner P, Weller M G, Niessner R, Lenke H, Knackmuss H-J. Reductive transformation of bound trinitrophenyl residues and free TNT during a bioremediation process analyzed by immunoassay. Environ Sci Technol. 1999;33:3421–3426.
-
- Agrawal A, Tratnyek P G. Reduction of nitroaromatic compounds by zero-valent iron metal. Environ Sci Technol. 1996;30:153–160.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

